Number Of Electrons In A 2p Orbital
Muz Play
Mar 27, 2025 · 6 min read
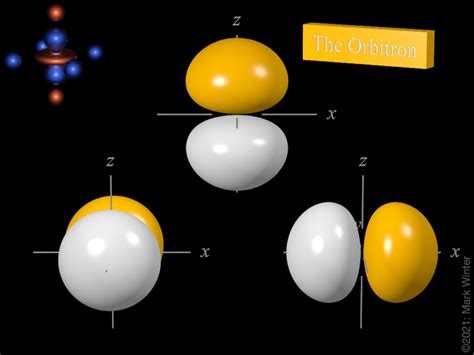
Table of Contents
Delving Deep into the 2p Orbital: How Many Electrons Can It Hold?
The seemingly simple question of how many electrons a 2p orbital can hold opens a door to a fascinating exploration of atomic structure, quantum mechanics, and the very nature of electron behavior within an atom. While the answer itself is straightforward, understanding why that answer is correct requires a deeper dive into the principles governing electron configuration. This article will thoroughly explore the 2p orbital, explaining its properties, its capacity for electrons, and the implications of its electron occupancy on atomic behavior.
Understanding Atomic Orbitals: A Foundation
Before we focus specifically on the 2p orbital, let's establish a foundational understanding of atomic orbitals. These orbitals aren't physical spaces in the same way we think of a room; instead, they are regions of space around the nucleus of an atom where there's a high probability of finding an electron. This probability is described by a wave function, a mathematical function derived from the Schrödinger equation, a cornerstone of quantum mechanics.
Quantum Numbers: Defining an Orbital
The specific properties of an orbital are defined by a set of four quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It's a positive integer (n = 1, 2, 3...). A higher 'n' value signifies a higher energy level and a larger orbital. For the 2p orbital, n = 2.
-
Azimuthal Quantum Number (l): This number determines the shape of the orbital and its angular momentum. It ranges from 0 to n-1. For l = 0, the orbital is an s orbital (spherical); for l = 1, it's a p orbital (dumbbell-shaped); l = 2 is a d orbital, and so on. The 2p orbital has l = 1.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It ranges from -l to +l, including 0. For a p orbital (l = 1), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes (px, py, and pz).
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum, or spin, of the electron. It can only have two values: +1/2 (spin up) or -1/2 (spin down). This quantum number is crucial for understanding the Pauli Exclusion Principle.
The 2p Orbital: Shape and Orientation
The 2p orbital, characterized by n=2 and l=1, possesses a unique dumbbell shape with two lobes of electron density on either side of the nucleus, separated by a nodal plane where the probability of finding an electron is zero. The three 2p orbitals (2px, 2py, 2pz) are oriented along the x, y, and z axes, respectively, forming a mutually perpendicular set. This spatial orientation is a direct consequence of the magnetic quantum number (ml).
The Pauli Exclusion Principle: The Key to Electron Capacity
The answer to "how many electrons can a 2p orbital hold?" hinges on the Pauli Exclusion Principle. This fundamental principle of quantum mechanics states that no two electrons in an atom can have the same set of four quantum numbers. In other words, each orbital can hold a maximum of two electrons, and these two electrons must have opposite spins (one spin up, one spin down).
Applying the Principle to the 2p Subshell
Since there are three 2p orbitals (2px, 2py, and 2pz), and each orbital can hold a maximum of two electrons, the total number of electrons that the 2p subshell can accommodate is 3 orbitals * 2 electrons/orbital = 6 electrons.
Electron Configuration and the 2p Orbital
The electron configuration of an atom describes how electrons are distributed among the various orbitals. For example, the element nitrogen (N), with an atomic number of 7, has the electron configuration 1s²2s²2p³. This means that the 1s orbital contains two electrons, the 2s orbital contains two electrons, and three of the six available 2p orbitals contain one electron each. The remaining 2p orbitals are unoccupied. Oxygen (O), with atomic number 8, has the electron configuration 1s²2s²2p⁴, filling four of the six available slots in the 2p subshell.
Beyond the Basics: Implications of 2p Electron Occupancy
The number of electrons in the 2p orbitals significantly impacts an atom's properties:
-
Chemical Bonding: The electrons in the 2p orbitals are valence electrons, meaning they participate in chemical bonding. The number of valence electrons determines the atom's reactivity and the types of bonds it can form (covalent, ionic). For example, the presence of three unpaired electrons in the nitrogen 2p orbitals allows nitrogen to form three covalent bonds, contributing to its versatility in forming various compounds.
-
Magnetic Properties: Atoms with unpaired electrons in their 2p orbitals exhibit paramagnetism, a type of magnetism where the atom is attracted to an external magnetic field. Atoms with all paired electrons in their 2p orbitals are diamagnetic, meaning they are slightly repelled by an external magnetic field.
-
Spectroscopic Properties: The energy differences between the 2p orbitals and other orbitals influence the absorption and emission spectra of atoms. These spectral lines are unique to each element and are used in spectroscopic techniques for identifying and analyzing substances.
-
Color: The interaction of light with electrons in the 2p orbitals (and other orbitals) can produce color. The specific wavelengths of light absorbed or emitted depend on the energy level differences between orbitals, resulting in the characteristic colors of various compounds.
Advanced Concepts: Hund's Rule and Orbital Filling
Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is due to electron-electron repulsion; it's energetically more favorable for electrons to occupy separate orbitals with parallel spins initially. Only after each orbital has a single electron will electrons begin to pair up with opposite spins.
Conclusion: The Significance of the 2p Orbital
The seemingly simple question of the number of electrons in a 2p orbital (two per orbital, six per subshell) unveils a wealth of information about atomic structure, quantum mechanics, and the properties of matter. Understanding the principles governing electron configuration, including the Pauli Exclusion Principle and Hund's Rule, is essential for grasping the behavior of atoms and their interactions to form molecules and materials with diverse properties. The 2p orbital, with its unique shape and capacity for electrons, plays a pivotal role in determining the chemical and physical properties of a vast array of elements and compounds, showcasing the fundamental importance of understanding its electron occupancy. This knowledge is vital in various scientific fields, from chemistry and materials science to physics and engineering. The seemingly simple answer—six electrons—opens a gateway to a much larger and fascinating understanding of the atomic world.
Latest Posts
Latest Posts
-
What Type Of Ion Will Calcium Form
Mar 30, 2025
-
The Atomic Mass Number Is Equal To
Mar 30, 2025
-
Where Does Dna Replication Take Place In A Eukaryotic Cell
Mar 30, 2025
-
Find Standard Matrix Of Linear Transformation
Mar 30, 2025
-
Is Delta H Products Minus Reactants
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In A 2p Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
