Number Of Valence Electrons Of Xenon
Muz Play
Mar 18, 2025 · 5 min read
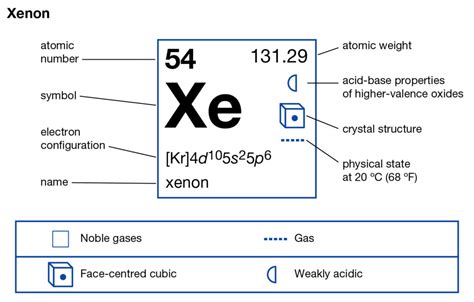
Table of Contents
Unveiling the Secrets of Xenon: Exploring its Valence Electrons
Xenon, a noble gas residing in Group 18 of the periodic table, has long captivated scientists with its unique properties. While its inert nature was once considered absolute, discoveries have revealed its capacity to form chemical compounds, challenging the traditional understanding of noble gas reactivity. Central to understanding this reactivity is the number of valence electrons xenon possesses. This article delves deep into the intricacies of xenon's electronic structure, exploring its valence electrons, their implications for chemical bonding, and the fascinating exceptions to the octet rule that xenon exhibits.
Understanding Valence Electrons: The Key to Reactivity
Before focusing specifically on xenon, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons is often predictable based on an element's position in the periodic table. For example, elements in Group 1 (alkali metals) typically have one valence electron, while Group 17 (halogens) have seven.
However, the noble gases, including xenon, present a unique case. They are characterized by a completely filled outermost electron shell, usually eight electrons (following the octet rule, a stable electron configuration), making them incredibly stable and unreactive. This is why they were initially considered inert.
Xenon's Electronic Configuration: A Closer Look
Xenon's atomic number is 54, indicating it has 54 electrons. Its electronic configuration is [Kr] 4d¹⁰ 5s² 5p⁶. The brackets [Kr] represent the configuration of krypton, a noble gas with a filled 3d and 4s subshell, demonstrating the noble gas core. The remaining electrons fill the 4d, 5s, and 5p subshells.
Crucially, the 5s² and 5p⁶ electrons constitute xenon's valence electrons. This sums up to a total of eight valence electrons. This complete octet is responsible for xenon's initial classification as an inert gas. The filled valence shell signifies exceptional stability, requiring significant energy input to disrupt its electron configuration and facilitate chemical bonding.
Challenging the Inert Nature: Xenon's Compound Formation
The discovery that xenon could form compounds marked a significant turning point in chemistry. While the octet rule generally holds true, it's not absolute, and noble gases, under specific conditions, can engage in chemical bonding. The formation of xenon compounds relies on overcoming the high ionization energy required to remove electrons from the stable, filled valence shell.
Factors Enabling Xenon's Reactivity
Several factors contribute to xenon's ability to form compounds:
- High pressure: Extreme pressures can force xenon atoms closer together, increasing the likelihood of electron interactions and bond formation.
- Highly electronegative atoms: Bonding with highly electronegative atoms, such as fluorine and oxygen, can help stabilize the resulting compounds by drawing electron density away from xenon.
- Strong oxidizing agents: Powerful oxidizing agents can further facilitate the oxidation of xenon, leading to compound formation.
Notable Xenon Compounds
Although the reactivity of xenon is significantly limited, several compounds have been synthesized, demonstrating the exceptions to the octet rule:
- Xenon difluoride (XeF₂): This compound showcases xenon's ability to expand its valence shell beyond eight electrons. The two fluorine atoms share electrons with xenon, resulting in a linear structure.
- Xenon tetrafluoride (XeF₄): Similar to XeF₂, XeF₄ involves xenon exceeding the octet rule. It adopts a square planar geometry.
- Xenon hexafluoride (XeF₆): The most fluorinated xenon compound, XeF₆, exhibits a distorted octahedral geometry.
- Xenon oxides: Xenon also forms oxides, although these are less stable than the fluorides.
Implications of Xenon's Valence Electrons: A Broader Perspective
The eight valence electrons of xenon have far-reaching implications beyond its ability to form compounds:
- Chemical inertness: The filled valence shell under normal conditions dictates its inherent chemical inertness. This inertness has led to applications in various fields, including lighting and medical imaging.
- Spectroscopic properties: The electronic configuration and valence electrons influence xenon's unique spectroscopic properties. The transitions between energy levels of its electrons determine its characteristic emission spectrum, crucial in applications such as lasers.
- Nuclear applications: Xenon isotopes are used in nuclear medicine and research, owing to their radioactive properties and distinctive nuclear characteristics.
The Expanding Realm of Noble Gas Chemistry: Beyond Xenon
While xenon's reactivity was a landmark discovery, it is not an isolated phenomenon. Other noble gases, particularly krypton and radon, have also been shown to form compounds, albeit under even more stringent conditions. These discoveries continue to reshape our understanding of chemical bonding and the periodic table's organization, highlighting the inherent complexity and fascinating exceptions that exist in the world of chemistry. Further research continues to push the boundaries of noble gas chemistry, revealing new possibilities and expanding our chemical knowledge.
Conclusion: The Significance of Xenon's Eight Valence Electrons
In conclusion, the eight valence electrons of xenon, although initially seemingly indicative of absolute inertness, are key to understanding its unique chemical behavior. While the octet rule serves as a useful guideline, xenon's ability to form compounds under specific conditions provides crucial insights into the flexibility of electron configurations and the complexities of chemical bonding. The study of xenon and its compounds has significantly enriched our understanding of chemistry, opening new avenues of research and application in various scientific fields. The fascinating journey of unraveling the secrets of xenon’s reactivity continues, highlighting the dynamic and ever-evolving nature of scientific discovery. Further research in this area undoubtedly promises more exciting revelations in the years to come, continuing to refine and expand our comprehension of the fundamental principles governing the behavior of matter.
Latest Posts
Latest Posts
-
How To Calculate Percent Of Water In A Hydrate
Mar 19, 2025
-
Shear Force Diagram Of Cantilever Beam
Mar 19, 2025
-
Mean And Variance Of Sample Mean
Mar 19, 2025
-
Predict The Product For The Reaction Shown
Mar 19, 2025
-
When Is Work Positive And Negative
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons Of Xenon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
