Oxidation And Reduction Always Occur Simultaneously
Muz Play
Mar 18, 2025 · 6 min read
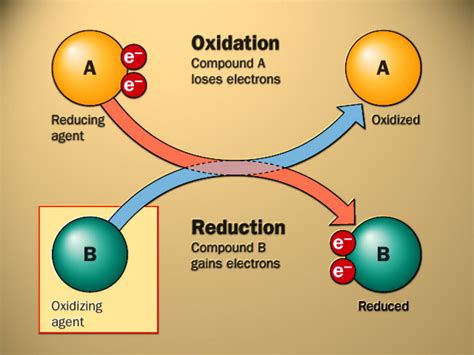
Table of Contents
Oxidation and Reduction: An Inseparable Duo
Oxidation and reduction, often abbreviated as redox reactions, are fundamental chemical processes that govern a vast array of natural phenomena and industrial applications. A crucial understanding of these reactions lies in recognizing their inseparable nature: oxidation and reduction always occur simultaneously. This principle, often overlooked in introductory chemistry, is key to comprehending the intricate electron transfers that underpin many chemical transformations. This article delves into the intricacies of redox reactions, explaining why they're intrinsically linked and exploring diverse examples showcasing this fundamental principle.
Understanding Oxidation and Reduction
Before delving into their simultaneous occurrence, let's define each process individually.
Oxidation: The Loss of Electrons
Oxidation, historically defined as the reaction with oxygen, is now more accurately described as the loss of electrons by an atom, molecule, or ion. This loss of electrons results in an increase in the oxidation state of the species involved. The species that loses electrons is termed the reducing agent because it causes the reduction of another species.
Examples of Oxidation:
- The rusting of iron: Iron (Fe) reacts with oxygen (O₂) in the presence of water to form iron(III) oxide (Fe₂O₃), commonly known as rust. Iron loses electrons and is oxidized, while oxygen gains electrons and is reduced.
- The combustion of methane: Methane (CH₄) burns in oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). Carbon in methane loses electrons and is oxidized, while oxygen gains electrons and is reduced.
- The reaction of magnesium with hydrochloric acid: Magnesium (Mg) reacts with hydrochloric acid (HCl) to produce magnesium chloride (MgCl₂) and hydrogen gas (H₂). Magnesium loses electrons and is oxidized, while hydrogen ions gain electrons and are reduced.
Reduction: The Gain of Electrons
Reduction, conversely, is defined as the gain of electrons by an atom, molecule, or ion. This gain of electrons leads to a decrease in the oxidation state of the species involved. The species that gains electrons is called the oxidizing agent because it causes the oxidation of another species.
Examples of Reduction:
- The formation of water from hydrogen and oxygen: Hydrogen (H₂) reacts with oxygen (O₂) to form water (H₂O). Oxygen gains electrons and is reduced, while hydrogen loses electrons and is oxidized.
- The electrolysis of water: The application of an electric current to water splits it into hydrogen and oxygen. Hydrogen ions gain electrons and are reduced to hydrogen gas, while oxygen atoms lose electrons and are oxidized to oxygen gas.
- The reduction of copper(II) ions: Copper(II) ions (Cu²⁺) can be reduced to copper metal (Cu) by the addition of a reducing agent such as zinc (Zn). Copper ions gain electrons and are reduced, while zinc loses electrons and is oxidized.
The Inseparable Dance: Why Oxidation and Reduction Occur Simultaneously
The key to understanding the simultaneous nature of oxidation and reduction lies in the conservation of charge. Electrons are not created or destroyed in chemical reactions; they are simply transferred from one species to another. Therefore, if one species loses electrons (oxidation), another species must gain those same electrons (reduction). This electron transfer is the essence of a redox reaction.
Imagine a seesaw. Oxidation is one side going down (losing electrons), and reduction is the other side going up (gaining electrons). You can't have one side go down without the other side going up; they are balanced. This balance is maintained by the equal number of electrons lost and gained.
The Redox Couple:
In every redox reaction, you have a redox couple. This couple consists of the oxidizing agent and the reducing agent. The oxidizing agent accepts electrons from the reducing agent, becoming reduced in the process, while the reducing agent donates electrons, becoming oxidized.
Half-Reactions:
To clearly illustrate the electron transfer, chemists often use half-reactions. These separate the oxidation and reduction processes, allowing for a clearer understanding of the electron transfer. For example, in the reaction between zinc (Zn) and copper(II) ions (Cu²⁺):
- Oxidation half-reaction: Zn(s) → Zn²⁺(aq) + 2e⁻ (Zinc loses two electrons)
- Reduction half-reaction: Cu²⁺(aq) + 2e⁻ → Cu(s) (Copper ions gain two electrons)
By adding these two half-reactions together, the electrons cancel out, resulting in the overall balanced redox reaction:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Identifying Redox Reactions: Oxidation Numbers
Determining whether a reaction is a redox reaction involves examining the oxidation numbers (or oxidation states) of the atoms involved. The oxidation number is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. A change in oxidation number indicates a redox reaction has occurred.
Rules for Assigning Oxidation Numbers:
- The oxidation number of an element in its free state is zero.
- The oxidation number of a monatomic ion is equal to its charge.
- The sum of oxidation numbers in a neutral molecule is zero.
- The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion.
- In most compounds, the oxidation number of hydrogen is +1, and the oxidation number of oxygen is -2 (except in peroxides, where it is -1).
Example:
Let's consider the reaction:
2Fe²⁺(aq) + Cl₂(aq) → 2Fe³⁺(aq) + 2Cl⁻(aq)
- Iron: The oxidation number of iron changes from +2 to +3, indicating oxidation (loss of electrons).
- Chlorine: The oxidation number of chlorine changes from 0 to -1, indicating reduction (gain of electrons).
Since oxidation and reduction both occur, this is a redox reaction.
Real-World Applications of Redox Reactions
Redox reactions are ubiquitous, playing vital roles in numerous processes:
Biological Systems:
- Cellular Respiration: The process that provides energy to living organisms relies heavily on redox reactions, involving the oxidation of glucose and the reduction of oxygen.
- Photosynthesis: Plants use light energy to convert carbon dioxide and water into glucose and oxygen, a process involving intricate redox reactions.
- Enzyme Catalysis: Many enzymes catalyze redox reactions, playing crucial roles in metabolism.
Industrial Processes:
- Metallurgy: Extraction of metals from their ores often involves redox reactions, such as the reduction of iron ore (Fe₂O₃) to iron metal (Fe) in a blast furnace.
- Battery Technology: Batteries function based on redox reactions, converting chemical energy into electrical energy.
- Corrosion Prevention: Understanding redox reactions is crucial for developing methods to prevent corrosion, a significant problem affecting many metal structures.
- Electroplating: Coating a metal object with a thin layer of another metal involves redox reactions, where the metal ions are reduced onto the object's surface.
Environmental Chemistry:
- Water Treatment: Redox reactions are used in water purification processes to remove pollutants.
- Atmospheric Chemistry: Redox reactions in the atmosphere contribute to the formation of smog and acid rain.
Conclusion: A Fundamental Principle
Oxidation and reduction are not independent processes; they are two sides of the same coin. The simultaneous nature of these reactions is a fundamental principle in chemistry, crucial for understanding electron transfer and the diverse processes dependent on it. By grasping this principle, we gain a deeper appreciation for the complexity and elegance of chemical reactions, their far-reaching implications in various fields, and the interconnectedness of the natural world. The study of redox reactions is not just an academic pursuit; it's a window into the very processes that shape our world. From the energy powering our bodies to the industries shaping our civilization, redox reactions play an indispensable role, highlighting their crucial importance in chemistry and beyond.
Latest Posts
Latest Posts
-
How To Add Formal Charges To Resonance Structures
Mar 19, 2025
-
What Are The Building Blocks Of A Carbohydrate
Mar 19, 2025
-
Substitution And Elimination Reactions Practice Problems
Mar 19, 2025
-
The Permanent Party Organization Consists Of
Mar 19, 2025
-
What Elements Are Most Likely To Become Anions
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Oxidation And Reduction Always Occur Simultaneously . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
