What Elements Are Most Likely To Become Anions
Muz Play
Mar 19, 2025 · 5 min read
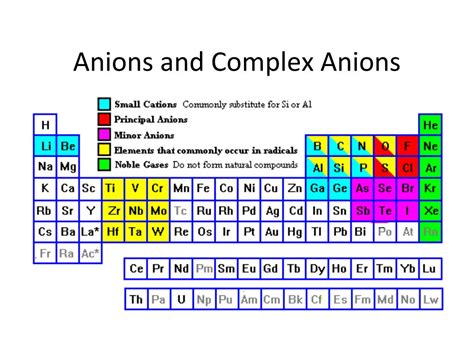
Table of Contents
What Elements Are Most Likely to Become Anions?
Understanding which elements readily form anions is crucial in chemistry. Anions, negatively charged ions, are fundamental building blocks in numerous compounds and play vital roles in various chemical processes. This article delves deep into the factors determining an element's propensity to form anions, exploring periodic trends, electronegativity, and the role of electron configuration. We'll examine specific groups of elements known for their anionic behavior, providing a comprehensive understanding of this important chemical concept.
The Driving Force: Electronegativity
The most significant factor influencing an element's tendency to become an anion is its electronegativity. Electronegativity measures an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity readily gain electrons to achieve a stable electron configuration, thus forming anions. This attraction is due to the positive charge of the nucleus and the effective nuclear charge experienced by the valence electrons. A higher effective nuclear charge means a stronger pull on electrons.
Periodic Trends in Electronegativity
Electronegativity isn't a constant property; it shows a clear trend across the periodic table. Generally:
-
Electronegativity increases across a period (left to right): As you move across a period, the number of protons in the nucleus increases, while the principal quantum number (shell level) remains the same. This leads to a stronger attraction for electrons, resulting in higher electronegativity.
-
Electronegativity decreases down a group (top to bottom): As you move down a group, the number of electron shells increases. The increased distance between the nucleus and valence electrons weakens the attractive force, leading to lower electronegativity.
This explains why elements in the upper right corner of the periodic table (excluding noble gases) have the highest electronegativity and are most likely to form anions.
The Octet Rule and Stable Electron Configurations
The pursuit of a stable electron configuration is the primary driver behind anion formation. The octet rule, while not universally applicable, states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell containing eight electrons (like noble gases). This stable configuration minimizes energy and increases stability.
Elements readily form anions when gaining electrons allows them to achieve this stable octet. For example, chlorine (Cl) has seven valence electrons. By gaining one electron, it achieves a stable octet and becomes a chloride ion (Cl⁻).
Groups Predominantly Forming Anions: A Deeper Dive
Certain groups on the periodic table are more predisposed to forming anions than others. Let's examine some prominent examples:
Group 17 (Halogens): Masters of Anion Formation
The halogens (fluorine, chlorine, bromine, iodine, and astatine) are exceptionally good at forming anions. With seven valence electrons, they only need to gain one electron to achieve a stable octet. Their high electronegativity further reinforces this tendency. Halogen anions (fluoride F⁻, chloride Cl⁻, bromide Br⁻, iodide I⁻) are ubiquitous in many compounds.
Specific examples and their importance:
-
Sodium Chloride (NaCl): Table salt, a crucial compound in biological systems and food preservation, consists of sodium cations (Na⁺) and chloride anions (Cl⁻).
-
Hydrochloric Acid (HCl): A strong acid, vital in industrial processes and digestion, is formed from hydrogen cations (H⁺) and chloride anions (Cl⁻).
-
Silver Halides: Silver halides (AgCl, AgBr, AgI) are light-sensitive compounds used in photography.
Group 16 (Chalcogens): Versatile Anion Formers
Chalcogens (oxygen, sulfur, selenium, tellurium, and polonium) have six valence electrons. They often gain two electrons to achieve a stable octet, forming anions with a -2 charge (oxide O²⁻, sulfide S²⁻, selenide Se²⁻, etc.). However, their behavior is more nuanced than halogens. They can exhibit variable oxidation states, sometimes forming compounds where they don't achieve a full octet.
Examples and their significance:
-
Oxides: Oxygen forms oxides with most elements, making it crucial for combustion and respiration. Many metal oxides are important in materials science.
-
Sulfides: Sulfides are abundant in minerals and play significant roles in geochemistry. Hydrogen sulfide (H₂S) is a toxic gas.
-
Sulfates: Sulfates (containing SO₄²⁻) are important anions found in many minerals and biological systems.
Group 15 (Pnictogens): Less Common, but Still Possible
Pnictogens (nitrogen, phosphorus, arsenic, antimony, and bismuth) have five valence electrons. While less prone to anion formation compared to halogens and chalcogens, they can form anions under specific conditions, often with a -3 charge (nitride N³⁻, phosphide P³⁻, etc.). Their larger size and lower electronegativity makes anion formation less favorable.
Examples and significance:
-
Nitrides: Nitrides are used in various applications, including ceramics and semiconductors.
-
Phosphides: Phosphides are found in some minerals and are less prevalent than oxides and sulfides.
Factors Influencing Anion Formation Beyond Electronegativity
While electronegativity is paramount, other factors can influence an element's ability to form anions:
-
Ionic Radius: Larger ionic radii can stabilize negative charge better, making anion formation more favorable.
-
Polarizability: Easily polarizable atoms are more likely to form anions as their electron cloud can be more easily distorted.
-
Reaction Conditions: The reaction environment (temperature, pressure, presence of other reactants) can significantly influence the formation of anions.
Exceptions and Beyond the Octet Rule
It's essential to note that the octet rule isn't absolute. Some elements can form stable compounds with more or fewer than eight electrons in their valence shell. Transition metals, for example, often form ions with various charges, not necessarily following the octet rule. Larger elements in later periods can also accommodate more than eight electrons due to the availability of d and f orbitals.
Conclusion
The propensity of an element to form an anion is primarily governed by its electronegativity and its pursuit of a stable electron configuration, often adhering to the octet rule. Halogens are the quintessential anion formers, readily gaining one electron to achieve a stable octet. Chalcogens frequently gain two electrons, while pnictogens form anions less readily. Understanding these trends and the underlying principles is crucial for comprehending chemical bonding, predicting chemical reactions, and interpreting the properties of countless compounds. While electronegativity provides a powerful framework, it's vital to consider factors like ionic radius, polarizability, and reaction conditions for a complete understanding of anion formation. The periodic table, with its predictable trends, serves as a valuable tool in predicting the anionic behavior of elements.
Latest Posts
Latest Posts
-
How To Identify Strong And Weak Acids And Bases
Mar 19, 2025
-
What Is The Building Block Monomer Of Nucleic Acids
Mar 19, 2025
-
Using Inverse Matrix To Solve System Of Equations
Mar 19, 2025
-
How Does Osmosis Affect Animal Cells
Mar 19, 2025
-
Algebra 1 Factor The Common Factor Out Of Each Expression
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Elements Are Most Likely To Become Anions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
