Patterns In The Periodic Table Of Elements
Muz Play
Mar 23, 2025 · 7 min read
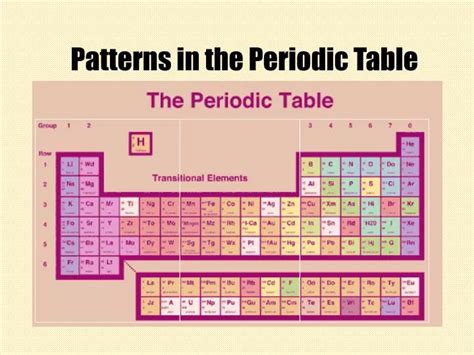
Table of Contents
Unveiling the Secrets: Patterns and Trends in the Periodic Table of Elements
The periodic table, a seemingly simple arrangement of elements, is a cornerstone of chemistry, physics, and materials science. More than just a list, it's a powerful tool revealing fundamental patterns and trends in the properties of elements, allowing scientists to predict the behavior of matter and design new materials. Understanding these patterns is crucial for comprehending the world around us, from the formation of stars to the development of life itself. This comprehensive exploration delves into the various patterns and trends embedded within the periodic table, highlighting their significance and applications.
The Organization: A Foundation for Understanding
The periodic table's genius lies in its organization. Elements are arranged in increasing order of their atomic number (number of protons), resulting in a systematic display of their properties. This organization is not arbitrary; it reflects the underlying quantum mechanical principles governing electron configuration.
Periods and Groups: The Building Blocks
The table is structured into periods (horizontal rows) and groups (vertical columns). Elements within a period share the same highest principal energy level (shell) for their electrons. As you move across a period, the number of electrons in the outermost shell (valence electrons) increases, leading to predictable changes in properties.
Groups, on the other hand, contain elements with the same number of valence electrons. This similarity in valence electron configuration leads to strikingly similar chemical behaviors. For example, Group 1 elements (alkali metals) all readily lose one electron to form +1 ions, exhibiting high reactivity. Similarly, Group 18 elements (noble gases) have full valence shells, making them exceptionally unreactive.
Block Structure: A Deeper Dive into Electron Configuration
Beyond periods and groups, the periodic table is further divided into blocks based on the type of atomic orbital being filled with electrons:
- s-block: Groups 1 and 2 (alkali metals and alkaline earth metals). These elements fill their outermost s orbitals.
- p-block: Groups 13 to 18. These elements fill their outermost p orbitals. This block includes a wide range of elements with diverse properties, including nonmetals, metalloids (semiconductors), and some metals.
- d-block: Groups 3 to 12 (transition metals). These elements are characterized by the filling of their inner d orbitals. Transition metals exhibit variable oxidation states and often form colored compounds.
- f-block: Lanthanides and actinides (placed separately at the bottom for aesthetic reasons). These elements fill their inner f orbitals, resulting in complex electronic configurations and unique magnetic properties.
Periodic Trends: Observing the Patterns
The periodic arrangement reveals several important periodic trends, systematic changes in properties across periods and down groups. These trends are crucial for predicting the chemical and physical behavior of elements.
Atomic Radius: Size Matters
Atomic radius, the distance from the nucleus to the outermost electron, shows a clear trend. Moving across a period, atomic radius generally decreases. This is due to an increasing effective nuclear charge (the net positive charge experienced by valence electrons) which pulls the electrons closer to the nucleus. Moving down a group, atomic radius generally increases. This is because additional electron shells are added, increasing the distance from the nucleus to the outermost electrons.
Ionization Energy: The Energy of Removal
Ionization energy is the energy required to remove an electron from a gaseous atom. Ionization energy generally increases across a period as the effective nuclear charge increases, making it harder to remove an electron. Ionization energy generally decreases down a group due to the increasing atomic radius and shielding effect (inner electrons reducing the effective nuclear charge on valence electrons).
Electronegativity: Sharing is Caring (or Not)
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period, as the effective nuclear charge increases. Electronegativity generally decreases down a group due to the increasing atomic radius and shielding effect. Elements with high electronegativity tend to attract electrons strongly, forming negatively charged ions or pulling electrons towards themselves in covalent bonds.
Electron Affinity: The Attraction of Electrons
Electron affinity is the energy change when an electron is added to a neutral atom. The trend is less straightforward than ionization energy and electronegativity, but generally, electron affinity increases across a period and decreases down a group, although exceptions exist. High electron affinity indicates a strong attraction for an additional electron.
Metallic Character: Metals, Nonmetals, and Metalloids
Metallic character refers to the properties associated with metals, such as conductivity, malleability, and ductility. Metallic character generally decreases across a period as the number of valence electrons increases, and the tendency to lose electrons diminishes. Metallic character generally increases down a group due to the decreasing ionization energy and the increasing atomic size. This trend leads to the division of elements into metals, nonmetals, and metalloids (semiconductors) with properties lying between metals and nonmetals.
Beyond the Basic Trends: More Complex Patterns
While the trends described above provide a fundamental understanding of periodic behavior, many nuances and exceptions exist. A comprehensive analysis requires considering additional factors:
Effective Nuclear Charge: The Unshielded Truth
The effective nuclear charge experienced by valence electrons plays a critical role in determining many periodic trends. This charge is the net positive charge felt by an electron after accounting for the shielding effect of inner electrons. The shielding effect, however, isn't perfect; different orbitals shield valence electrons to varying degrees, impacting the trends.
Electron-Electron Repulsion: The Crowded Space
As more electrons occupy the same shell, electron-electron repulsion increases, slightly counteracting the effect of increasing effective nuclear charge. This effect is especially noticeable in larger atoms and ions.
Orbital Penetration: Getting Closer to the Nucleus
Different orbitals penetrate closer to the nucleus than others. Electrons in orbitals that penetrate more effectively experience a stronger effective nuclear charge and have lower energy. This effect influences ionization energies and other properties.
Applications of Periodic Trends: From Theory to Practice
Understanding periodic trends is not just an academic exercise; it has far-reaching applications in various fields:
Materials Science: Designing New Materials
By understanding the periodic trends, scientists can predict the properties of new materials and design them with specific characteristics. For example, the trend in electronegativity allows scientists to predict the type of bonding in compounds and their resulting properties like melting point, conductivity, and reactivity. This is critical in the development of new alloys, semiconductors, superconductors, and other advanced materials.
Chemical Reactions: Predicting Reactivity
The trends in ionization energy, electronegativity, and electron affinity allow chemists to predict the reactivity of elements and compounds. This knowledge is essential for designing chemical reactions, optimizing reaction conditions, and developing new synthetic methods.
Environmental Science: Understanding Pollutant Behavior
Periodic trends help us understand the environmental behavior of elements. For instance, knowledge of the solubility and reactivity of different metals helps in assessing the toxicity and environmental impact of pollutants.
Medicine and Biology: The Role of Trace Elements
Many elements are essential for biological processes, and understanding their properties based on periodic trends is crucial in medicine and biology. Trace elements like iron, zinc, and copper play vital roles in various enzymatic reactions and metabolic pathways.
Conclusion: The Enduring Legacy of the Periodic Table
The periodic table is not merely a classification system; it’s a testament to the underlying order and predictability of the universe. The patterns and trends observed within it are essential tools for understanding the behavior of matter at the atomic and molecular level. From predicting the reactivity of elements to designing new materials with desired properties, the periodic table continues to be an indispensable guide in the fields of chemistry, physics, materials science, and beyond. Its enduring legacy lies in its ability to unite seemingly disparate facts into a coherent and elegant framework, revealing the deep connections between the elements and their profound influence on the world around us. The exploration of periodic trends is an ongoing journey, with new discoveries continually enriching our understanding of this fundamental tool of science.
Latest Posts
Latest Posts
-
Does Reduction Happen At The Cathode
Mar 25, 2025
-
What Are The Units For Wavelength
Mar 25, 2025
-
What Instrument Is Used For Measuring Mass
Mar 25, 2025
-
Electric Field Between Two Opposite Charges
Mar 25, 2025
-
In A Molecule With Covalent Bonding
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Patterns In The Periodic Table Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
