Periodic Table Labeled With Metals Nonmetals And Metalloids
Muz Play
Mar 23, 2025 · 6 min read
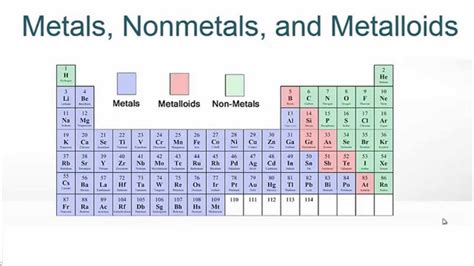
Table of Contents
The Periodic Table: A Colorful Guide to Metals, Nonmetals, and Metalloids
The periodic table, a cornerstone of chemistry, organizes chemical elements in a structured format, revealing patterns in their properties and behaviors. This intricate arrangement isn't just a random collection; it's a carefully crafted map that unlocks the secrets of matter. One of the most fundamental ways we categorize elements within this table is by their classification as metals, nonmetals, or metalloids. Understanding these distinctions is crucial to grasping the fundamental principles of chemistry and the diverse properties of the elements themselves.
Metals: The Stars of the Periodic Table
Metals dominate the periodic table, occupying the left and center regions. Their defining characteristics stem from their atomic structure: they readily lose electrons, forming positive ions (cations). This characteristic explains many of their shared properties.
Key Properties of Metals:
- Conductivity: Metals are exceptional conductors of both heat and electricity. This property is due to the free movement of electrons within their metallic lattice structure. This is why metals are ubiquitous in electrical wiring and heat sinks.
- Malleability and Ductility: Metals can be hammered into sheets (malleability) and drawn into wires (ductility). This is because their atoms can slide past each other without breaking the metallic bonds. Think of gold being beaten into incredibly thin sheets for gilding, or copper being stretched into thin wires.
- Luster: Metals typically possess a shiny or lustrous appearance. This is a result of their interaction with light; the free electrons reflect light effectively. This property makes them desirable for decorative purposes, in jewelry and other aesthetic applications.
- High Tensile Strength: Many metals exhibit high tensile strength, meaning they can withstand significant pulling forces before breaking. This strength is vital in construction, engineering, and manufacturing applications.
- High Density: Most metals possess relatively high densities compared to nonmetals. This property is due to the close packing of their atoms within the metallic lattice.
- High Melting and Boiling Points: Generally, metals have high melting and boiling points. This reflects the strong metallic bonds holding their atoms together. Exceptions exist, of course, such as mercury, which is a liquid at room temperature.
Examples of Common Metals and Their Uses:
- Iron (Fe): Used in steel, construction, and machinery due to its strength and abundance. It's a fundamental element in many industrial processes.
- Aluminum (Al): Lightweight and corrosion-resistant, it's used in aircraft, beverage cans, and numerous consumer products. Its recyclability makes it environmentally friendly.
- Copper (Cu): An excellent conductor of electricity, copper is widely used in electrical wiring, plumbing, and electronics.
- Gold (Au): Highly valued for its inertness, malleability, and beauty, gold is used in jewelry, electronics, and investments.
- Silver (Ag): Another excellent conductor, silver finds applications in electronics, photography, and silverware.
Nonmetals: A Diverse Group with Varied Properties
Nonmetals occupy the upper right-hand corner of the periodic table. Unlike metals, they tend to gain electrons, forming negative ions (anions). This fundamental difference leads to contrasting physical and chemical properties.
Key Properties of Nonmetals:
- Poor Conductivity: Nonmetals are generally poor conductors of heat and electricity. Their electrons are tightly bound to their atoms, limiting electron mobility.
- Brittle: Nonmetals are typically brittle and lack the malleability and ductility seen in metals. They tend to shatter when subjected to stress.
- Dull Appearance: Nonmetals generally lack the luster of metals; they often appear dull or have a non-reflective surface.
- Low Density: Nonmetals generally have lower densities than metals.
- Low Melting and Boiling Points: Many nonmetals have relatively low melting and boiling points.
- Variety in States of Matter: Nonmetals exist in all three states of matter at room temperature: solid (carbon), liquid (bromine), and gas (oxygen).
Examples of Common Nonmetals and Their Uses:
- Oxygen (O): Essential for respiration and combustion, oxygen is a vital component of the atmosphere.
- Carbon (C): Forms the basis of organic chemistry, carbon exists in various allotropes, including diamond and graphite. It's crucial in countless materials.
- Nitrogen (N): A major component of the atmosphere, nitrogen is used in fertilizers and various industrial processes.
- Chlorine (Cl): Used in water purification and various industrial chemical processes.
- Hydrogen (H): The lightest element, hydrogen is used as a fuel and in many chemical processes.
Metalloids: The Bridge Between Metals and Nonmetals
Metalloids, also known as semimetals, form a fascinating bridge between metals and nonmetals. They are located along the zigzag line separating metals and nonmetals on the periodic table. Their properties are intermediate between those of metals and nonmetals, exhibiting a blend of characteristics.
Key Properties of Metalloids:
- Semiconductor Behavior: This is the defining characteristic of metalloids. They are semiconductors of electricity, meaning their conductivity can be controlled by varying temperature, light exposure, or doping (adding impurities). This property is critical in modern electronics.
- Variable Conductivity: Their conductivity lies between metals (high conductivity) and nonmetals (low conductivity).
- Brittle Nature: Metalloids are generally brittle, like nonmetals.
- Metallic Luster (Sometimes): Some metalloids display a metallic luster, while others may appear dull.
- Intermediate Melting and Boiling Points: Their melting and boiling points fall between those of metals and nonmetals.
Examples of Common Metalloids and Their Uses:
- Silicon (Si): The most prominent metalloid, silicon is a fundamental component of semiconductors used in computer chips, solar cells, and other electronics. It’s the backbone of the modern electronics industry.
- Germanium (Ge): Also used in semiconductors, germanium was historically important in transistors but has been largely replaced by silicon in many applications.
- Arsenic (As): Used in some semiconductors and also as a doping agent in other semiconductor materials.
- Antimony (Sb): Used in alloys to enhance their hardness and strength.
- Tellurium (Te): Used in solar cells and some specialized alloys.
The Importance of Understanding Metal, Nonmetal, and Metalloid Classification
Classifying elements as metals, nonmetals, or metalloids is essential for several reasons:
- Predicting Properties: Knowing an element's classification helps predict its physical and chemical properties. This allows scientists to design materials with specific characteristics.
- Designing Materials: The unique properties of metals, nonmetals, and metalloids allow for the creation of a vast array of materials with tailored characteristics, from superconductors to strong alloys.
- Understanding Chemical Reactions: The reactivity of an element is closely tied to its classification. Understanding this allows us to predict how elements will interact with each other.
- Technological Advancements: The properties of these element classes are crucial to countless technological advancements, from electronics to medicine.
Conclusion: A Periodic Journey of Discovery
The periodic table is more than just a chart; it's a powerful tool for understanding the building blocks of our universe. The classification of elements into metals, nonmetals, and metalloids provides a fundamental framework for understanding their properties and behaviors. As we continue to explore the intricacies of the periodic table, we unlock new possibilities for materials science, technological innovation, and a deeper understanding of the world around us. The seemingly simple act of categorizing elements is a testament to the power of organization and the remarkable interconnectedness of the chemical world. Further investigation into the specific properties of individual elements within these categories will only enrich our understanding of their diverse applications and contributions to our world.
Latest Posts
Latest Posts
-
How Does The Digestive System Interact With The Muscular System
Mar 24, 2025
-
Osteocytes Sit In Small Chambers Called
Mar 24, 2025
-
What Do Roman Numerals Mean In Chemistry
Mar 24, 2025
-
The Energy Investment Steps Of Glycolysis Use
Mar 24, 2025
-
Are Metals On The Right Side O The Periodic Table
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Labeled With Metals Nonmetals And Metalloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
