Periodic Table With Lanthanides And Actinides Inserted
Muz Play
Mar 21, 2025 · 6 min read
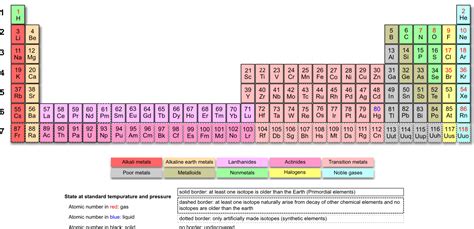
Table of Contents
The Periodic Table: A Comprehensive Guide Including Lanthanides and Actinides
The periodic table is a cornerstone of chemistry, a beautifully organized arrangement of elements that reveals fundamental relationships between their properties. At first glance, it appears relatively straightforward, but a deeper dive reveals the intricate complexities and fascinating stories woven into its structure, especially when considering the inclusion of the lanthanides and actinides. This article delves into the history, structure, and significance of the periodic table, with a specific focus on the integration and unique characteristics of these two series of elements.
The Genesis of the Periodic Table: A History of Organization
The journey to the modern periodic table was a collaborative effort spanning decades, involving numerous scientists and their observations. Early attempts focused on classifying elements based on atomic weight, noticing recurring patterns in their properties. Johann Wolfgang Döbereiner's triads, grouping elements with similar properties in sets of three, represented an early step. Later, John Newlands' Law of Octaves attempted to organize elements based on the repetition of properties every eighth element, analogous to musical octaves. These early efforts, while imperfect, laid the groundwork for more comprehensive systems.
The true breakthrough arrived independently with Dmitri Mendeleev and Lothar Meyer. Mendeleev, famously, arranged elements based on their atomic weight and recurring chemical properties. Crucially, he left gaps in his table, predicting the existence and properties of undiscovered elements. These predictions, which were later verified, solidified the power and predictive capabilities of his periodic table. Meyer, concurrently, developed a similar arrangement using atomic volume, further reinforcing the underlying principle of periodic recurrence.
The Structure of the Periodic Table: Rows, Columns, and Blocks
The modern periodic table organizes 118 elements based on their atomic number (number of protons), electron configuration, and recurring chemical properties. The table is arranged in rows called periods and columns called groups.
-
Periods: Each period represents a principal energy level, with elements in the same period having the same number of electron shells. The number of elements in each period varies, reflecting the increasing complexity of electron configurations.
-
Groups: Elements in the same group share similar chemical properties due to having the same number of valence electrons (electrons in the outermost shell). These similar properties influence their reactivity and bonding behavior. Groups are further classified into main-group elements (representative elements), transition metals, and inner transition metals (lanthanides and actinides).
-
Blocks: Elements are categorized into blocks (s, p, d, and f) based on the subshells that their valence electrons occupy. This provides additional insights into their electronic structure and properties. For example, the s-block elements are generally highly reactive metals, while the p-block elements exhibit a broader range of properties.
Lanthanides and Actinides: The Inner Transition Metals
The lanthanides (also called rare earth elements) and actinides form the f-block elements, situated at the bottom of the periodic table. Their placement reflects their unique electronic structure, with valence electrons filling the 4f subshell (lanthanides) and 5f subshell (actinides). This differentiates them from other elements and accounts for their specific chemical and physical characteristics.
Lanthanides: A Series of Similar Properties
The lanthanides, from cerium (Ce) to lutetium (Lu), are characterized by their remarkably similar chemical properties. This similarity stems from their comparable electronic configurations, with valence electrons primarily residing in the 4f subshell, which is shielded from the outer electrons. This shielding effect reduces the influence of the 4f electrons on chemical reactivity, resulting in a high degree of similarity in their behavior.
Despite their chemical similarities, subtle differences exist. These differences are largely attributable to the lanthanide contraction, a phenomenon where the ionic radii of lanthanide ions decrease across the series. This contraction influences their chemical behavior and coordination chemistry, and explains minor variations in their reactivity.
Actinides: Radioactivity and Diverse Properties
The actinides, from thorium (Th) to lawrencium (Lr), differ significantly from lanthanides in that most are radioactive. Their radioactivity stems from the instability of their large nuclei, leading to alpha decay and other forms of radioactive emission. This radioactivity influences their chemical behavior and makes them challenging to study.
Unlike the lanthanides, the actinides exhibit a wider range of oxidation states and chemical properties. This diversity arises from the participation of both 5f and 6d electrons in chemical bonding, leading to more complex chemical behavior. This complexity, coupled with their radioactivity, makes their study more intricate than that of the lanthanides.
The Importance of Including Lanthanides and Actinides
The inclusion of lanthanides and actinides in the periodic table is crucial for a comprehensive understanding of the elemental landscape. These elements, while sometimes overlooked, play vital roles in various fields:
-
Technology: Lanthanides are essential components in many modern technologies, including high-strength magnets, catalysts in petroleum refining, and components in lighting and display technologies.
-
Nuclear Energy: Actinides, particularly uranium and plutonium, are vital in nuclear power generation and nuclear weapons. Understanding their properties is paramount for safe and responsible management of nuclear materials.
-
Medical Applications: Some actinides are used in medical imaging and radiation therapy. Careful handling and administration are necessary due to their radioactivity.
-
Research and Development: Both lanthanides and actinides continue to be the subject of intense research, exploring their potential applications in various fields and pushing the boundaries of chemical and physical understanding.
The Future of the Periodic Table
The periodic table is not a static entity; it evolves with the discovery of new elements. Synthetic elements, created through nuclear reactions, continue to expand the table. The discovery and characterization of these elements contribute to our understanding of nuclear physics and the limits of elemental stability.
Moreover, ongoing research into the properties and applications of existing elements, particularly the lanthanides and actinides, unveils new possibilities and opens doors to advancements in various technologies. The periodic table, therefore, remains a dynamic tool, reflecting our ever-growing knowledge of matter and its fundamental constituents.
Conclusion: A Testament to Scientific Discovery
The periodic table, encompassing lanthanides and actinides, stands as a testament to the power of scientific observation, meticulous experimentation, and collaborative efforts. It is a powerful tool for organizing, predicting, and understanding the behavior of elements, providing a framework for countless scientific advancements. The ongoing exploration of its nuances, especially the intriguing properties of the inner transition metals, ensures that the periodic table will continue to be a central focus in chemical research and technological innovation for years to come. Its story is a continuous narrative of scientific discovery, a testament to human curiosity and the pursuit of knowledge. The periodic table, with its intricate tapestry of elements, is more than just a chart; it's a dynamic map charting the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
Non Homogeneous System Of Linear Equations
Mar 28, 2025
-
Diversifiable Risk And Non Diversifiable Risk
Mar 28, 2025
-
Normal Boiling Point On Phase Diagram
Mar 28, 2025
-
What Are Alpha Beta And Gamma
Mar 28, 2025
-
What Is The Function Of The Atrioventricular Valves
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table With Lanthanides And Actinides Inserted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
