Normal Boiling Point On Phase Diagram
Muz Play
Mar 28, 2025 · 5 min read
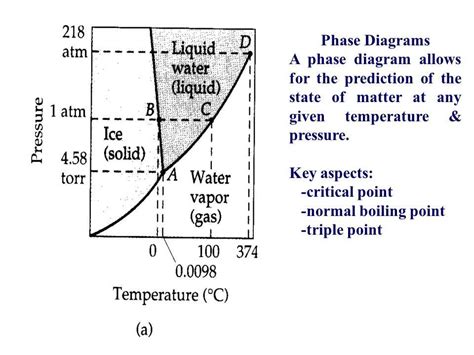
Table of Contents
Normal Boiling Point on a Phase Diagram: A Comprehensive Guide
The normal boiling point is a fundamental concept in chemistry and physics, crucial for understanding the behavior of substances under different conditions. This comprehensive guide will delve into the meaning of the normal boiling point, its representation on a phase diagram, the factors influencing it, and its significance in various applications. We'll explore this concept in detail, offering a robust understanding for students and enthusiasts alike.
Understanding the Normal Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. At this point, the liquid transitions rapidly into the gaseous phase, forming bubbles within the liquid itself. Crucially, the boiling point is temperature-dependent, varying with changes in external pressure.
The normal boiling point, however, is specifically defined as the boiling point of a liquid when the external pressure is exactly one standard atmosphere (1 atm or 101.325 kPa). This standardized pressure ensures a consistent and comparable measurement across different experiments and substances. It's the temperature at which a substance boils under "normal" atmospheric conditions at sea level.
Phase Diagrams: A Visual Representation
Phase diagrams are invaluable tools for visualizing the physical states of a substance—solid, liquid, and gas—under varying conditions of temperature and pressure. They provide a concise graphical representation of the phase transitions (melting, boiling, sublimation, etc.) a substance undergoes.
A typical phase diagram features three distinct regions representing the solid, liquid, and gas phases. The lines separating these regions denote the conditions under which two phases coexist in equilibrium. The line separating the liquid and gas phases is particularly relevant to our discussion of the normal boiling point.
Locating the Normal Boiling Point on a Phase Diagram
On a phase diagram, the normal boiling point is found at the intersection of the liquid-gas equilibrium line and the horizontal line representing a pressure of 1 atm. This point indicates the temperature at which the liquid will boil when the pressure is at standard atmospheric pressure.
Imagine this: Trace a horizontal line across the phase diagram at 1 atm. Where this line intersects the liquid-gas equilibrium curve, you've located the normal boiling point. The temperature corresponding to this intersection point is the normal boiling point of the substance.
Factors Affecting the Normal Boiling Point
Several factors influence the normal boiling point of a substance. These factors are intricately linked to the intermolecular forces within the substance:
1. Intermolecular Forces
Stronger intermolecular forces (like hydrogen bonding, dipole-dipole interactions, and London dispersion forces) require more energy to overcome, thus resulting in a higher normal boiling point. Substances with strong intermolecular forces hold their molecules together more tightly, requiring a higher temperature to break these bonds and transition to the gaseous phase. For instance, water, with its strong hydrogen bonds, has a relatively high boiling point compared to substances with weaker intermolecular forces.
2. Molecular Weight
Generally, higher molecular weight leads to a higher normal boiling point. Larger molecules have more electrons, resulting in stronger London dispersion forces. These stronger forces require more energy to overcome, hence the increased boiling point. This trend is particularly noticeable in homologous series of organic compounds.
3. Molecular Shape and Branching
The shape and branching of a molecule can also influence the boiling point. Linear molecules tend to have higher boiling points than branched molecules with the same molecular weight because linear molecules can pack more closely together, leading to stronger intermolecular interactions. Branched molecules have more steric hindrance, hindering close packing and weakening the intermolecular forces.
4. Hydrogen Bonding
Hydrogen bonding, a particularly strong type of dipole-dipole interaction, significantly impacts the boiling point. Substances capable of forming hydrogen bonds (like water, alcohols, and amines) exhibit substantially higher boiling points than comparable molecules without hydrogen bonding capabilities.
Significance and Applications of the Normal Boiling Point
The normal boiling point is a crucial physical property with numerous applications across various fields:
1. Purification and Separation
The normal boiling point is fundamental in techniques like distillation, which separates components of a mixture based on their differing boiling points. In fractional distillation, substances with different boiling points are separated by carefully controlling the temperature to vaporize and condense each component sequentially.
2. Chemical Reactions
Knowing the normal boiling point helps in designing and controlling chemical reactions. Many reactions require specific temperature ranges, and understanding the boiling point of solvents and reactants is critical for ensuring the reaction proceeds safely and efficiently.
3. Material Science
In material science, the normal boiling point aids in characterizing and selecting materials for specific applications. The boiling point influences a material's volatility, stability at high temperatures, and suitability for various processing techniques.
4. Environmental Science
Understanding boiling points is crucial in environmental science, particularly when assessing the volatility and potential environmental impact of different substances. Volatile compounds with low boiling points can easily evaporate and contribute to air pollution.
5. Food Science
In food science, the boiling point is relevant to cooking and food preservation. Understanding boiling points helps in controlling cooking temperatures and ensuring proper food processing techniques.
Advanced Considerations: Deviations from Ideal Behavior
While the concepts discussed above provide a good general understanding, it's important to acknowledge that real-world substances may deviate from ideal behavior. Factors such as:
- Association in the liquid phase: Some liquids form aggregates or clusters of molecules, influencing their boiling behavior.
- Non-ideal mixtures: Interactions between different components in a mixture can affect their boiling points compared to their individual pure components.
- Effects of impurities: The presence of impurities can slightly alter the boiling point of a liquid.
These deviations are often small, but they highlight the complexities of real-world systems and underscore the need for careful experimental determination of boiling points in specific situations.
Conclusion: The Importance of the Normal Boiling Point
The normal boiling point, a seemingly simple concept, is a powerful tool for understanding and predicting the behavior of substances. Its representation on phase diagrams, the factors influencing its value, and its diverse applications across various fields emphasize its significance in science and technology. Understanding the normal boiling point is key to mastering many essential chemical and physical processes and contributes to advancements in diverse fields. This comprehensive overview provides a solid foundation for further exploration into the fascinating world of phase transitions and material properties.
Latest Posts
Latest Posts
-
What Type Of Population Density Dependence Focuses On Abiotic Factors
Mar 31, 2025
-
What Are Two Divisions Of The Skeleton
Mar 31, 2025
-
When Is It Acceptable To Use A Personnel Platform
Mar 31, 2025
-
Packing Efficiency Of Body Centered Cubic
Mar 31, 2025
-
What Is Reversible And Irreversible Process In Thermodynamics
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Normal Boiling Point On Phase Diagram . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
