Ph Value Of Milk Of Magnesia
Muz Play
Mar 29, 2025 · 5 min read
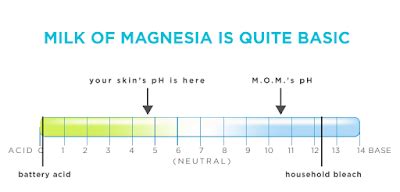
Table of Contents
Understanding the pH Value of Milk of Magnesia: A Comprehensive Guide
Milk of magnesia, a common over-the-counter antacid and laxative, is a suspension of magnesium hydroxide in water. Its effectiveness stems largely from its alkaline nature, characterized by its pH value. This article delves deep into the pH of milk of magnesia, exploring its implications for health, usage, and potential interactions. We'll also examine how the pH is measured and the factors influencing its stability.
What is pH and Why is it Important?
Before diving into the specifics of milk of magnesia, it's crucial to understand the concept of pH. pH is a scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. The scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are alkaline or basic. Each whole number change represents a tenfold change in acidity or alkalinity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
The pH of a substance profoundly impacts its chemical properties and biological activity. In the human body, maintaining a precise pH balance is vital for various physiological processes. Even slight deviations from the optimal pH range can disrupt enzyme function, cellular processes, and overall health.
The pH of Milk of Magnesia: A Deep Dive
Milk of magnesia, primarily composed of magnesium hydroxide (Mg(OH)₂), exhibits a highly alkaline pH, typically ranging from 10 to 11. This high pH is the key to its antacid and laxative properties. The alkaline nature of milk of magnesia helps neutralize stomach acid, providing relief from heartburn and indigestion. Its alkalinity also stimulates bowel movements by drawing water into the intestines, softening the stool and promoting easier passage.
Factors Influencing pH Variation
While the typical pH range is 10-11, several factors can subtly influence the precise pH of a specific milk of magnesia product:
-
Manufacturing process: Slight variations in the manufacturing process can affect the final pH. This includes factors such as the purity of the magnesium hydroxide used, the water quality, and the mixing process.
-
Storage conditions: Exposure to air and changes in temperature can impact the pH over time. Improper storage can lead to degradation and slight changes in the solution's alkalinity.
-
Expiration date: As milk of magnesia ages, its pH may fluctuate slightly. Using expired milk of magnesia can lead to inconsistent results and potentially reduced effectiveness. Always check the expiration date before use.
-
Brand variations: While the overall pH range remains consistent across brands, slight differences might exist due to variations in manufacturing techniques and formulations.
How is the pH of Milk of Magnesia Measured?
The pH of milk of magnesia is typically measured using a pH meter or pH indicator.
-
pH meter: This electronic instrument provides a precise digital reading of the pH by measuring the electrical potential difference between a reference electrode and a sensing electrode immersed in the solution. pH meters offer high accuracy and are commonly used in laboratories and quality control settings.
-
pH indicator: These are chemical substances that change color depending on the pH of the solution. While less precise than a pH meter, pH indicators offer a quick and visual way to assess the approximate pH. Litmus paper is a common example of a pH indicator. However, for precise pH determination of milk of magnesia, a pH meter is preferred.
Milk of Magnesia's Action: Neutralization and Osmotic Effects
The high pH of milk of magnesia is central to its dual action as an antacid and a laxative.
Antacid Action
When ingested, milk of magnesia reacts with stomach acid (primarily hydrochloric acid, HCl), neutralizing its acidity through a simple acid-base reaction:
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
This reaction produces magnesium chloride (MgCl₂) and water, effectively reducing the concentration of hydrochloric acid and alleviating heartburn symptoms.
Laxative Action
Milk of magnesia's laxative effect is primarily due to its osmotic properties. Its high pH and magnesium content draw water into the intestines, increasing stool bulk and softening the stool. This facilitates easier bowel movements and relieves constipation.
Safety Considerations and Potential Interactions
While generally safe, milk of magnesia should be used judiciously. Excessive consumption can lead to:
-
Diarrhea: Overuse can cause significant diarrhea, leading to dehydration and electrolyte imbalances.
-
Magnesium toxicity: High levels of magnesium in the bloodstream can cause various adverse effects, including nausea, vomiting, muscle weakness, and even cardiac arrhythmias. This is particularly concerning for individuals with kidney problems, as the kidneys play a crucial role in magnesium excretion.
Potential drug interactions: Milk of magnesia can interact with certain medications, including:
-
Tetracyclines: Milk of magnesia can reduce the absorption of tetracycline antibiotics.
-
Digoxin: It can increase the risk of digoxin toxicity.
Therefore, it's crucial to consult a doctor or pharmacist before using milk of magnesia if you are taking other medications or have any underlying health conditions.
Milk of Magnesia and its Use in Different pH Environments
The highly alkaline pH of milk of magnesia makes it unsuitable for some applications. For example, its use in acidic environments might lead to unwanted reactions and reduce its effectiveness. Conversely, in highly alkaline environments, its impact might be minimal. Its efficacy is maximized in situations where it needs to neutralize acidity, specifically in the context of the stomach's acidic environment.
Conclusion: pH as a Key to Understanding Milk of Magnesia's Function
The pH value of milk of magnesia, typically ranging from 10 to 11, is a fundamental property determining its efficacy as both an antacid and a laxative. Understanding this pH and its implications for neutralization and osmotic effects is crucial for safe and effective use. Remember that while generally safe when used as directed, excessive consumption or use in conjunction with other medications can lead to adverse effects. Always consult a healthcare professional if you have any concerns or underlying health conditions before using milk of magnesia. The information provided here is for educational purposes only and should not be considered medical advice. Always consult with a qualified healthcare provider for any health concerns or before making any decisions related to your health or treatment.
Latest Posts
Latest Posts
-
Four Ways To Represent A Function
Mar 31, 2025
-
What Is The Functional Unit Of Heredity
Mar 31, 2025
-
Cracking The Code Of Life Answer Key
Mar 31, 2025
-
Delta H Is Negative Exothermic Or Endothermic
Mar 31, 2025
-
Raising And Lowering Operators Angular Momentum
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Ph Value Of Milk Of Magnesia . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
