Polarity Lead To High Specific Heat
Muz Play
Mar 27, 2025 · 7 min read
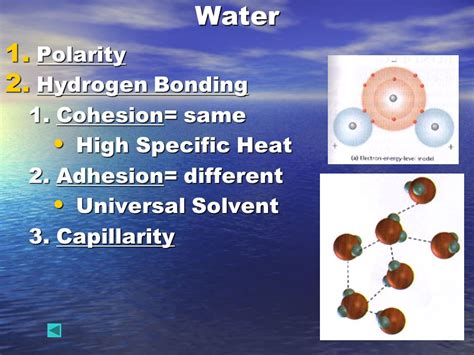
Table of Contents
Polarity Leads to High Specific Heat: Understanding the Molecular Dance
Specific heat, a fundamental property of matter, quantifies the amount of heat energy required to raise the temperature of a substance by a certain degree. Water, famously, boasts an exceptionally high specific heat. This seemingly simple fact underpins countless natural processes and technological applications, from regulating Earth's climate to enabling life itself. The key to understanding water's high specific heat lies in the polarity of its molecules. This article delves into the intricate molecular interactions that explain this phenomenon, exploring the role of hydrogen bonding and its implications for various systems.
The Polar Nature of Water: A Microscopic Marvel
Water (H₂O) isn't just a simple combination of hydrogen and oxygen; it's a marvel of molecular architecture. The oxygen atom, being more electronegative, attracts the shared electrons in the covalent bonds more strongly than the hydrogen atoms. This unequal sharing of electrons creates a polar molecule, meaning it possesses a slightly negative end (near the oxygen) and a slightly positive end (near the hydrogens). This polarity is the genesis of many of water's unique properties, including its high specific heat.
Understanding Electronegativity and its Impact
Electronegativity, a crucial concept in chemistry, describes an atom's ability to attract electrons within a chemical bond. Oxygen's higher electronegativity compared to hydrogen is the driving force behind the polar nature of the water molecule. This difference in electronegativity isn't trivial; it's the fundamental reason why water molecules interact with each other in such a unique and significant way.
The Dance of Hydrogen Bonds: A Powerful Intermolecular Force
The polarity of water molecules leads to the formation of hydrogen bonds. These are relatively strong intermolecular forces that occur between the slightly positive hydrogen atom of one water molecule and the slightly negative oxygen atom of another. These bonds aren't as strong as covalent bonds (the bonds within the water molecule itself), but they are significantly stronger than other intermolecular forces like van der Waals forces.
The Strength in Numbers: The Cumulative Effect of Hydrogen Bonds
While individual hydrogen bonds are relatively weak, the sheer number of them in a sample of water is enormous. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating an intricate, three-dimensional network. This extensive network is the key to water's unusually high specific heat.
High Specific Heat: Breaking and Reforming Bonds
To raise the temperature of a substance, you must increase the kinetic energy of its molecules – essentially, make them move faster. In substances with weak intermolecular forces, this requires relatively little energy. However, in water, a significant portion of the added energy is used to break and reform hydrogen bonds, rather than solely increasing the kinetic energy of the molecules.
Energy Absorption: A Molecular Tug-of-War
When heat is added to water, some of the energy goes into increasing molecular motion, but a substantial portion is consumed in disrupting the hydrogen bond network. The molecules need to overcome the attractive forces holding them together before they can move more freely. This energy absorption slows down the rate at which the temperature increases, resulting in a high specific heat.
Energy Release: Rebuilding the Network
Conversely, when water cools down, the hydrogen bonds reform, releasing energy in the process. This energy release moderates the rate of temperature decrease. This constant breaking and reforming of hydrogen bonds act as a buffer, preventing drastic temperature fluctuations.
The Significance of Water's High Specific Heat
The high specific heat of water has profound implications for various systems, both natural and man-made:
-
Climate Regulation: Water's high specific heat helps moderate Earth's climate. Large bodies of water absorb vast amounts of solar energy without experiencing significant temperature increases. This prevents extreme temperature fluctuations, creating a more stable environment.
-
Temperature Stability in Organisms: Water makes up a significant portion of living organisms. Its high specific heat helps maintain stable internal temperatures, protecting cells from damage caused by rapid temperature changes. This is vital for the functioning of biological systems.
-
Industrial Applications: The high specific heat of water makes it an excellent coolant in many industrial processes, such as power plants and manufacturing facilities. Its ability to absorb large amounts of heat without a significant temperature increase makes it highly efficient for heat transfer.
-
Ocean Currents and Weather Patterns: The high specific heat of ocean water significantly influences global weather patterns and ocean currents. The heat capacity of the oceans acts as a massive heat reservoir, influencing atmospheric temperatures and precipitation patterns across the globe.
-
Dissolving Power: The polarity of water, along with its high specific heat, contributes to its remarkable ability to dissolve a wide range of substances. This is crucial for biological processes, as it facilitates the transport of nutrients and other essential molecules within organisms.
Comparing Water's Specific Heat to Other Substances
Compared to many other substances, water's specific heat is remarkably high. For example, the specific heat of ethanol is significantly lower than that of water, meaning that less energy is required to raise the temperature of ethanol by the same amount. This difference is directly attributable to the presence and strength of hydrogen bonding in water, which is considerably weaker or absent in many other liquids. The absence of strong intermolecular forces in substances like metals generally results in much lower specific heat capacities.
The Role of Molecular Structure and Intermolecular Forces
The specific heat of a substance is intrinsically linked to its molecular structure and the strength of the intermolecular forces between its molecules. Substances with strong intermolecular forces, like water, tend to have higher specific heats than those with weaker forces. This is because a larger portion of the added energy is used to overcome these intermolecular attractions rather than solely increasing molecular kinetic energy.
Beyond Water: Polarity and Specific Heat in Other Substances
While water is the quintessential example, the relationship between polarity and high specific heat extends to other polar substances. For instance, ammonia (NH₃) is also a polar molecule capable of forming hydrogen bonds, albeit fewer than water. Consequently, ammonia possesses a relatively high specific heat compared to non-polar substances, though still lower than water. The specific heat of various polar liquids is directly influenced by the strength and number of hydrogen bonds they form, and other intermolecular forces present.
Factors Influencing Specific Heat in Polar Substances
Several factors contribute to the specific heat of polar substances:
-
Strength of Hydrogen Bonds: Stronger hydrogen bonds necessitate more energy to break them, leading to a higher specific heat.
-
Number of Hydrogen Bonds: A greater number of hydrogen bonds per molecule further increases the energy required to disrupt the network, resulting in a higher specific heat.
-
Molecular Weight: Heavier molecules generally require more energy to increase their kinetic energy, potentially contributing to a higher specific heat.
-
Other Intermolecular Forces: The presence of other intermolecular forces, such as dipole-dipole interactions or van der Waals forces, can also influence the overall specific heat.
Conclusion: The Crucial Role of Polarity in Determining Specific Heat
The high specific heat of water, and other polar substances, is a direct consequence of the polarity of their molecules and the resulting formation of hydrogen bonds. These strong intermolecular forces require significant energy to break, leading to a high capacity for absorbing heat without substantial temperature changes. This remarkable property plays a crucial role in various natural phenomena and has far-reaching implications in numerous technological applications. Understanding this intricate molecular dance sheds light on the remarkable properties of water and other polar substances, highlighting the fundamental connection between molecular structure, intermolecular forces, and macroscopic properties. Further research continues to unravel the complexities of these interactions and their influence on a wide range of scientific and engineering disciplines.
Latest Posts
Latest Posts
-
What Is The Difference Between Open And Closed Circulatory Systems
Mar 30, 2025
-
Which Substance Is An Example Of A Colloid
Mar 30, 2025
-
When An Atom Loses An Electron It Becomes
Mar 30, 2025
-
Differential Scanning Calorimetry Glass Transition Temperature
Mar 30, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Polarity Lead To High Specific Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
