Properties Of Alkali Metals And Alkaline Earth Metals
Muz Play
Mar 23, 2025 · 6 min read
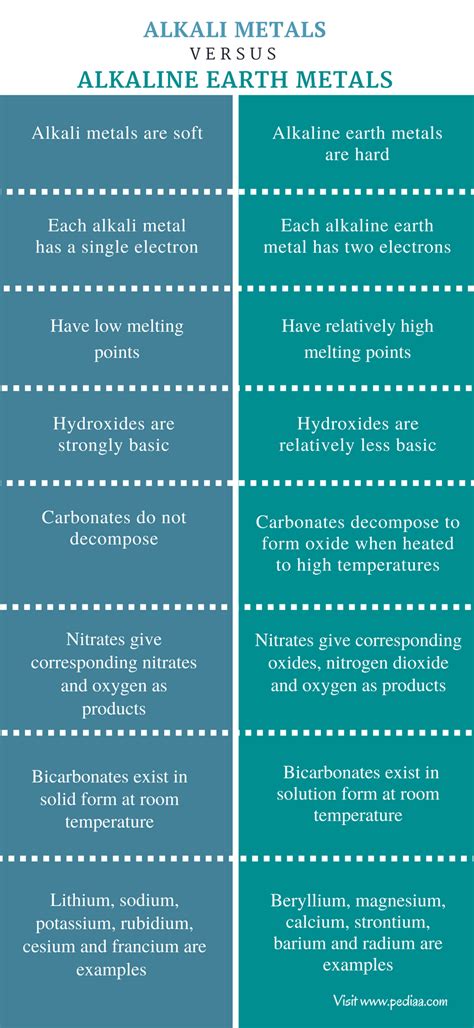
Table of Contents
Properties of Alkali Metals and Alkaline Earth Metals: A Deep Dive
Alkali and alkaline earth metals, residing in Groups 1 and 2 of the periodic table respectively, represent fascinating families of elements exhibiting distinct yet interconnected characteristics. Understanding their properties is crucial for appreciating their diverse applications in various fields, from everyday life to advanced technologies. This comprehensive exploration delves into the unique attributes of these groups, highlighting their similarities, differences, and the underlying reasons for their behavior.
Alkali Metals: Reactivity Personified
Alkali metals – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) – are defined by their single valence electron. This seemingly simple feature is the source of their remarkable reactivity and distinctive properties.
1. Physical Properties:
- Low Density: Alkali metals are remarkably light. Lithium, the lightest metal, floats on water. This low density stems from their relatively large atomic radii and weak metallic bonding.
- Low Melting and Boiling Points: The weak metallic bonding within alkali metals translates to relatively low melting and boiling points. They are soft enough to be cut with a knife, showcasing their weak interatomic forces.
- High Electrical and Thermal Conductivity: The loosely held valence electron allows for excellent electrical and thermal conductivity. These free electrons can easily move throughout the metallic lattice, facilitating the transfer of both heat and electricity.
- Metallic Luster: Freshly cut alkali metals exhibit a characteristic silvery-white metallic luster, though this quickly tarnishes upon exposure to air due to oxidation.
- Ductility and Malleability: Alkali metals are highly ductile (can be drawn into wires) and malleable (can be hammered into sheets), further illustrating their weak metallic bonds and the ease with which their atoms can be rearranged.
2. Chemical Properties:
- Extremely Reactive: The single valence electron is readily lost, forming a +1 ion. This makes alkali metals exceptionally reactive, particularly with water and halogens. The reactivity increases dramatically as you move down the group, with cesium being the most reactive.
- Reaction with Water: Alkali metals react vigorously with water, producing hydrogen gas and a metal hydroxide. The reaction becomes increasingly violent down the group. Lithium reacts steadily, sodium quite vigorously, and potassium, rubidium, and cesium react explosively.
- Reaction with Halogens: Alkali metals react readily with halogens (Group 17 elements) to form ionic halides (e.g., NaCl, KCl). These ionic compounds are characterized by high melting points and strong ionic bonds.
- Formation of Ionic Compounds: Due to their tendency to lose one electron, alkali metals almost always form ionic compounds with non-metals. The resulting ionic bonds are strong, leading to high melting and boiling points in these compounds.
- Oxidation States: Alkali metals always exhibit a +1 oxidation state in their compounds, reflecting the loss of their single valence electron.
3. Applications:
Alkali metals and their compounds find a wide range of applications, including:
- Lithium: Used in rechargeable batteries (lithium-ion batteries), lubricating greases, and certain ceramics. Lithium compounds have applications in medicine (treating bipolar disorder) and as a component in some aluminum alloys.
- Sodium: Sodium chloride (table salt) is essential for life. Sodium is also used in sodium vapor lamps, sodium hydroxide (lye) in cleaning products, and sodium bicarbonate (baking soda) in cooking and cleaning.
- Potassium: Crucial for plant growth and human health. Potassium compounds are used in fertilizers and various industrial applications.
- Rubidium and Cesium: Used in atomic clocks and other specialized applications where their unique atomic properties are utilized.
Alkaline Earth Metals: A Step Up in Reactivity
Alkaline earth metals – beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) – are located in Group 2 of the periodic table. They possess two valence electrons, leading to a set of properties that are similar to, but distinct from, those of alkali metals.
1. Physical Properties:
- Higher Density than Alkali Metals: Alkaline earth metals are denser than alkali metals, reflecting their stronger metallic bonding and smaller atomic radii.
- Higher Melting and Boiling Points than Alkali Metals: The stronger metallic bonding in alkaline earth metals results in higher melting and boiling points compared to alkali metals.
- Good Electrical and Thermal Conductivity: While not as high as alkali metals, alkaline earth metals still exhibit good electrical and thermal conductivity due to their valence electrons.
- Metallic Luster: Similar to alkali metals, they possess a silvery-white metallic luster when freshly cut.
- Less Ductile and Malleable than Alkali Metals: They are less ductile and malleable than alkali metals, reflecting the stronger metallic bonding.
2. Chemical Properties:
- Reactive, but Less Than Alkali Metals: Alkaline earth metals are reactive, but less so than alkali metals. This is because they need to lose two electrons to achieve a stable octet, requiring more energy.
- Reaction with Water: The reactivity with water increases down the group. Beryllium doesn't react with water, magnesium reacts slowly, while calcium, strontium, and barium react more vigorously, producing hydrogen gas and a metal hydroxide.
- Reaction with Oxygen: Alkaline earth metals react with oxygen to form metal oxides (e.g., MgO, CaO). These oxides are often basic in nature, reacting with water to form hydroxides.
- Formation of Ionic Compounds: Like alkali metals, they primarily form ionic compounds with non-metals. However, the +2 oxidation state leads to stronger ionic bonds and higher melting points.
- Oxidation States: Alkaline earth metals generally exhibit a +2 oxidation state, corresponding to the loss of their two valence electrons. However, beryllium can sometimes form covalent compounds.
3. Applications:
Alkaline earth metals and their compounds find widespread use in various applications:
- Magnesium: Used in lightweight alloys (e.g., in aircraft and automobiles), in flash photography, and as a reducing agent in certain chemical processes. Magnesium hydroxide is used as an antacid.
- Calcium: Essential for bone formation and various biological processes. Calcium carbonate (limestone) is used extensively in construction, and calcium sulfate (gypsum) in plaster and cement.
- Beryllium: Used in specialized alloys due to its high strength-to-weight ratio and unique properties. However, beryllium is toxic and requires careful handling.
- Strontium: Used in fireworks to produce red color, and certain strontium compounds find applications in medical imaging.
- Barium: Barium sulfate is used as a contrast agent in medical X-rays.
Comparing Alkali and Alkaline Earth Metals: A Summary Table
| Property | Alkali Metals | Alkaline Earth Metals |
|---|---|---|
| Valence Electrons | 1 | 2 |
| Reactivity | Very high, increases down the group | High, increases down the group but less than alkali metals |
| Density | Low | Higher than alkali metals |
| Melting Point | Low | Higher than alkali metals |
| Oxidation State | +1 | +2 |
| Reaction with Water | Vigorous, increases down the group | Less vigorous, increases down the group |
| Ionic Compound Formation | Primarily ionic | Primarily ionic |
| Ductility/Malleability | High | Lower than alkali metals |
Conclusion: A Periodic Tale of Reactivity
Alkali and alkaline earth metals, while neighbors on the periodic table, showcase a fascinating contrast in their properties. Their reactivity, driven by their valence electron configurations, defines their behavior and determines their applications in various fields. From the ubiquitous sodium in our salt shakers to the lightweight magnesium in our vehicles, these elements play crucial roles in modern society. A deeper understanding of their properties allows us to appreciate their importance and potential for future innovations. Further research continues to explore new applications of these remarkable elements and their compounds. The ongoing investigation into their properties serves as a testament to the ever-evolving landscape of chemical science. The remarkable diversity within these seemingly simple groups showcases the power and intricacy of the periodic table.
Latest Posts
Latest Posts
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
-
Rusting Of Iron Is Chemical Or Physical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Properties Of Alkali Metals And Alkaline Earth Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
