Provide The Correct Systematic Name For The Compound Shown Here
Muz Play
Apr 01, 2025 · 6 min read
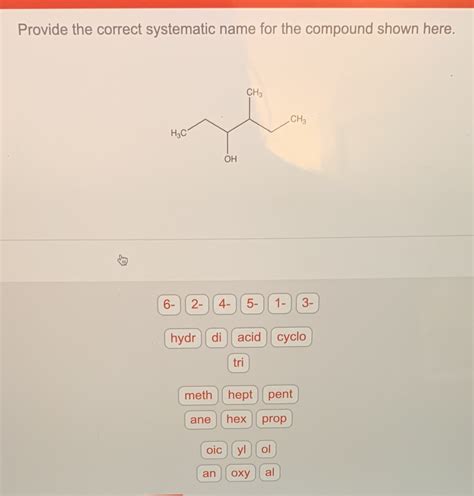
Table of Contents
Decoding Chemical Structures: A Deep Dive into Systematic Nomenclature
The ability to correctly name chemical compounds is fundamental to chemistry. It allows chemists worldwide to unambiguously communicate about specific molecules, regardless of language or background. This article will delve into the systematic naming of chemical compounds, providing a comprehensive guide to understanding and applying the rules of nomenclature, specifically focusing on how to determine the correct systematic name for a given chemical structure. While a specific structure isn't provided in the prompt, this article will equip you with the knowledge to tackle any given structure. We'll cover alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more, illustrating the principles with various examples.
Understanding the IUPAC System
The International Union of Pure and Applied Chemistry (IUPAC) is the globally recognized authority for chemical nomenclature. The IUPAC system is a logical and systematic approach, ensuring that each unique chemical structure has a single, unambiguous name. This avoids confusion and promotes clear communication within the scientific community. The system relies on a series of rules and priorities to assign names correctly. Mastering these rules is crucial for accurate and efficient communication within the field of chemistry.
Nomenclature of Alkanes: The Foundation
Alkanes, the simplest hydrocarbons, form the basis for understanding more complex organic molecules. Their systematic names follow a straightforward pattern:
-
Identify the longest continuous carbon chain: This chain forms the parent alkane name. Count the number of carbons; one carbon is methane, two is ethane, three is propane, four is butane, five is pentane, and so on.
-
Number the carbon atoms: Start numbering from the end that gives the substituents the lowest possible numbers.
-
Identify and name substituents: Substituents are groups of atoms attached to the main carbon chain. Methyl (-CH₃), ethyl (-CH₂CH₃), propyl (-CH₂CH₂CH₃), etc., are common substituents.
-
Combine the information: List the substituents alphabetically, preceding their position numbers on the parent chain. Use hyphens to separate numbers and names, and commas to separate numbers. The parent alkane name comes last.
Example: Consider a compound with a five-carbon chain and a methyl group on the second carbon.
- Longest chain: Pentane (5 carbons)
- Substituent: Methyl
- Position: 2-methyl
- Combined Name: 2-Methylpentane
Nomenclature of Alkenes and Alkynes: Locating the Unsaturation
Alkenes contain carbon-carbon double bonds (C=C), while alkynes contain carbon-carbon triple bonds (C≡C). The naming convention incorporates these functionalities:
-
Identify the longest carbon chain containing the multiple bond: This forms the parent name. The suffix "-ene" is used for alkenes, and "-yne" for alkynes.
-
Number the carbon atoms: Begin numbering from the end closest to the multiple bond, giving the multiple bond the lowest possible number. The number indicating the position of the double or triple bond is placed before the parent name.
-
Substituents are named and positioned as in alkanes: Alphabetical ordering and numerical positioning remain crucial.
Example: A compound with four carbons and a double bond between the second and third carbons.
- Longest chain: Butene (4 carbons, double bond)
- Double bond position: 2-butene
- Combined Name: 2-Butene
Alcohols, Aldehydes, Ketones, and Carboxylic Acids: Functional Group Priority
These classes of compounds contain functional groups that take priority in the naming system.
-
Alcohols (-OH): The suffix "-ol" is added to the parent alkane name. The position of the hydroxyl group (-OH) is indicated by a number.
-
Aldehydes (-CHO): The suffix "-al" is added. The aldehyde group is always at the end of the chain, so no number is needed.
-
Ketones (C=O): The suffix "-one" is used. The position of the carbonyl group (C=O) is indicated by a number.
-
Carboxylic Acids (-COOH): The suffix "-oic acid" is used. The carboxyl group (-COOH) is always at the end of the chain, so no number is needed.
Examples:
- Propan-2-ol: A three-carbon chain with a hydroxyl group on the second carbon.
- Propanal: A three-carbon chain with an aldehyde group at the end.
- Propan-2-one: A three-carbon chain with a ketone group on the second carbon.
- Propanoic acid: A three-carbon chain with a carboxylic acid group at the end.
Compounds with Multiple Functional Groups: Establishing Priority
When a molecule contains multiple functional groups, a priority order dictates the naming scheme. Carboxylic acids have the highest priority, followed by aldehydes, ketones, alcohols, alkenes, and alkynes. The highest priority functional group determines the suffix, while other functional groups are treated as substituents. Specific prefixes are used for these substituents (e.g., hydroxy- for alcohol, oxo- for ketone).
Example: A molecule containing both a hydroxyl group and a ketone group. The ketone would have priority, and the hydroxyl group would be treated as a hydroxy substituent.
Cyclic Compounds: Dealing with Rings
Cyclic compounds (rings) require a different approach.
-
Identify the parent ring: The parent name is based on the number of carbons in the ring (e.g., cyclopropane, cyclobutane, cyclopentane).
-
Number the ring carbons: Begin numbering at a substituent and proceed in the direction that gives the next substituent the lowest possible number.
-
Name and position substituents: This follows the same rules as for acyclic compounds.
Example: A cyclohexane ring with a methyl group on carbon 1 and an ethyl group on carbon 3. The name would be 1-methyl-3-ethylcyclohexane.
Stereochemistry: Indicating Spatial Arrangement
Stereochemistry involves the three-dimensional arrangement of atoms in a molecule. This information is crucial for accurately describing the compound. This often involves the use of prefixes such as cis and trans (for geometric isomers) or R and S (for chiral centers). The precise rules for incorporating stereochemical information are complex and beyond the scope of this introductory article, but understanding its significance is vital.
Advanced Nomenclature: Tackling Complexity
The principles discussed here provide a strong foundation for naming organic compounds. However, more complex molecules with multiple functional groups, rings, and stereochemical features may require a more in-depth understanding of IUPAC rules and conventions. Specialized textbooks and online resources are available to address these advanced scenarios.
Practical Application and Resources
The best way to master chemical nomenclature is through practice. Working through numerous examples, starting with simple molecules and gradually increasing complexity, will build your skills and confidence. Many online resources, including interactive exercises and nomenclature quizzes, can assist in this process. Furthermore, consulting chemical handbooks and databases can provide additional support and confirmation.
Conclusion: The Power of Precise Chemical Communication
Systematic nomenclature is a critical aspect of chemistry, fostering clear and unambiguous communication. Understanding and applying the IUPAC rules enables chemists to describe any molecule precisely, regardless of its complexity. This article has provided a foundational understanding of the principles involved, equipping you with the tools to correctly name a vast range of organic compounds. Remember that practice is key, and there are numerous resources available to aid your learning journey. With continued practice and a thorough understanding of the rules, you’ll be well-equipped to navigate the world of chemical structures and their systematic names.
Latest Posts
Latest Posts
-
Write In Standard Form The Equation Of Each Line
Apr 02, 2025
-
What Does Fad Stand For In Biology
Apr 02, 2025
-
The Outermost Layer Of The Heart Is Called The
Apr 02, 2025
-
Which Of The Following Are Channels Of Nonverbal Communication
Apr 02, 2025
-
Express The Interval As An Inequality
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Provide The Correct Systematic Name For The Compound Shown Here . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
