Question Violet What Is The Multiplicity Of The Methyl Peak
Muz Play
Mar 15, 2025 · 6 min read
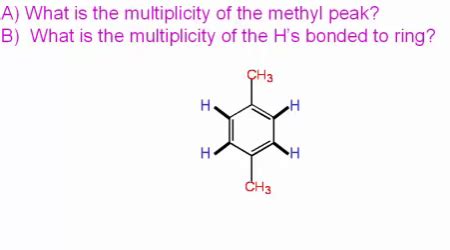
Table of Contents
Deconstructing the Methyl Peak: Understanding Multiplicity in NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used extensively in chemistry to determine the structure of organic molecules. One of the key aspects of interpreting NMR spectra is understanding the multiplicity of signals, specifically the splitting patterns observed due to spin-spin coupling. This article delves deep into the multiplicity of the methyl peak, focusing on the factors that influence its appearance and how to interpret it effectively. We will address the question, "What is the multiplicity of the methyl peak?", exploring various scenarios and complexities.
Understanding the Basics of Spin-Spin Coupling
Before we tackle the complexities of methyl peak multiplicity, it's crucial to establish a solid understanding of spin-spin coupling. This phenomenon arises from the interaction of the magnetic moments of neighboring nuclei. The magnetic field experienced by a nucleus is subtly altered by the spins of its neighboring nuclei, leading to a splitting of its NMR signal.
The n+1 Rule: A fundamental rule of thumb for predicting the multiplicity of a signal is the n+1 rule. Here, 'n' represents the number of equivalent neighboring protons. Therefore, a proton with 'n' equivalent neighboring protons will appear as a multiplet with n+1 peaks.
Example: A proton with three equivalent neighboring protons (n=3) will appear as a quartet (3+1=4 peaks).
However, the n+1 rule is a simplification and doesn't account for all scenarios. Several factors can influence the observed multiplicity, leading to deviations from the n+1 prediction.
Factors Affecting Methyl Peak Multiplicity
The multiplicity of a methyl peak (–CH3) depends primarily on the number and nature of its neighboring protons. Let's explore various scenarios:
1. No Neighboring Protons: If the methyl group has no neighboring protons, the methyl peak will appear as a singlet (one peak). This is because there's no spin-spin coupling influencing its signal.
Example: Consider a molecule like acetone (CH3)2CO. The methyl protons experience no coupling with other protons and appear as a singlet.
2. One Neighboring Proton: If the methyl group has one neighboring proton, the peak will appear as a doublet (two peaks). The intensity ratio of the two peaks will be approximately 1:1.
Example: Consider a molecule with a –CH2–CH3 fragment. The methyl protons (CH3) will couple with the single neighboring proton on the CH2 group, resulting in a doublet.
3. Two Neighboring Protons: When a methyl group is adjacent to a methylene group (–CH2–), it will typically show a triplet (three peaks) with an approximate intensity ratio of 1:2:1.
Example: Propane (CH3CH2CH3) provides a classic example. The methyl protons couple with the two neighboring methylene protons, resulting in a triplet.
4. Three Neighboring Protons: A methyl group next to a methine group (–CH–) will show a quartet (four peaks) with an intensity ratio of 1:3:3:1.
Example: Isobutane [(CH3)3CH] demonstrates this. The methyl protons couple with three equivalent protons on the central carbon, resulting in a quartet.
5. More Complex Scenarios: When considering more complex molecules, the multiplicity can become more intricate. The presence of multiple sets of neighboring protons with different chemical shifts can lead to more complex splitting patterns. Moreover, factors like the coupling constant (J value) and the chemical shift differences between coupled protons influence the appearance of the multiplet.
Coupling Constants (J values): The magnitude of the splitting, expressed as the coupling constant (J value), is crucial. Similar J values often lead to overlapping peaks, resulting in a seemingly simplified multiplet.
Deviations from the n+1 Rule:
Several factors can cause deviations from the simple n+1 rule:
-
Strong Coupling: When the chemical shift difference between coupled protons is small, compared to their coupling constant (J), the n+1 rule breaks down, leading to more complex splitting patterns. This is known as strong coupling.
-
Virtual Coupling: This occurs when two protons are not directly coupled but exhibit apparent coupling through a chain of coupled protons. This can lead to unexpected splitting patterns.
-
Diastereotopic Protons: If the neighboring protons are diastereotopic (not chemically equivalent), the n+1 rule doesn't directly apply. The multiplicity will depend on the specific coupling constants between the methyl protons and each of the diastereotopic protons.
-
Chemical Exchange: Rapid exchange of protons (e.g., through acid-base reactions or through hydrogen bonding) can average out the coupling effects, leading to simplification of the multiplicity.
Analyzing Methyl Peak Multiplicity in Practice:
Analyzing the multiplicity of a methyl peak involves careful observation and interpretation of the NMR spectrum. Here are key steps:
-
Identify the methyl peak: This usually appears at a chemical shift around 0.9-1.5 ppm (though this can vary depending on the molecular environment).
-
Determine the number of neighboring protons: Observe the splitting pattern. A singlet suggests no neighboring protons, a doublet suggests one, a triplet suggests two, and so on.
-
Consider the J values: The spacing between the peaks within a multiplet provides information about the coupling constant (J value). Similar J values may indicate coupling to equivalent protons.
-
Check for any deviations from the n+1 rule: If the multiplicity is more complex than expected, consider factors like strong coupling, virtual coupling, diastereotopic protons, or chemical exchange.
-
Integrate the peaks: The integrated area under each peak provides information about the number of protons responsible for the signal. The integration of a methyl peak should ideally correspond to three protons.
Advanced Techniques and Applications:
-
2D NMR: Techniques such as COSY (Correlation Spectroscopy) and HSQC (Heteronuclear Single Quantum Correlation) can provide more detailed information about coupling networks, aiding in the unambiguous assignment of methyl peaks and their multiplicity.
-
Computational Chemistry: Modern computational tools can predict NMR spectra, providing valuable support in the interpretation of complex multiplicity patterns. This can be particularly useful in the analysis of novel molecules where experimental data might be scarce.
Conclusion:
The multiplicity of the methyl peak in NMR spectroscopy provides valuable structural information about the molecule. While the n+1 rule serves as a useful guideline, understanding the factors that influence spin-spin coupling and potential deviations from this rule is crucial for accurate interpretation. By carefully analyzing the splitting patterns, integration values, and coupling constants, researchers can effectively decipher the structural details of organic molecules and answer the question, "What is the multiplicity of the methyl peak?" accurately and comprehensively. Mastering the interpretation of these subtle details ultimately enhances one's proficiency in using NMR spectroscopy as a powerful tool in chemical analysis. Remember to always correlate your NMR findings with other spectroscopic data to achieve a complete and robust structural elucidation.
Latest Posts
Latest Posts
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Question Violet What Is The Multiplicity Of The Methyl Peak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
