Reactvity With A Base A Physical Or Chemical Property
Muz Play
Mar 16, 2025 · 6 min read
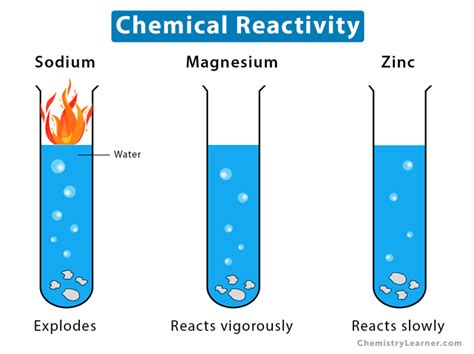
Table of Contents
Reactivity with a Base: A Physical or Chemical Property?
Reactivity is a fundamental concept in chemistry, describing the tendency of a substance to undergo a chemical change. While often discussed in the context of acids and bases, understanding its nature—whether it's a physical or chemical property—requires a closer look. This article will delve into the reactivity of substances with bases, exploring the underlying chemical processes and clarifying its classification as a chemical property.
Understanding Reactivity
Before diving into base reactivity, let's establish a clear understanding of what reactivity means. Reactivity isn't about how a substance looks or feels; it's about its inherent ability to transform into something else through chemical reactions. This transformation involves the breaking and forming of chemical bonds, leading to a change in the chemical composition of the substance. This is fundamentally different from physical properties, which describe characteristics that can be observed or measured without changing the substance's composition.
For example, the melting point of ice is a physical property; melting ice changes its state but not its composition. However, the reaction of sodium metal with water is a chemical property; a new substance (sodium hydroxide and hydrogen gas) is formed, fundamentally altering the chemical composition.
Reactivity with Bases: A Chemical Affair
Reactivity with a base, much like reactivity with an acid, is unequivocally a chemical property. This is because the interaction always results in a chemical change. The base's reactivity stems from its ability to donate hydroxide ions (OH⁻) or accept protons (H⁺), leading to several different types of chemical reactions. Let's examine some common examples:
1. Neutralization Reactions
One of the most familiar reactions involving bases is neutralization. This occurs when an acid reacts with a base, producing salt and water. The reaction involves the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base, forming water (H₂O).
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic neutralization reaction:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
In this reaction, the hydrochloric acid, a strong acid, reacts completely with the sodium hydroxide, a strong base. The products are sodium chloride (table salt), a neutral salt, and water. The change in chemical composition – from reactants (acid and base) to products (salt and water) – definitively establishes this as a chemical reaction, and therefore, reactivity with the base (NaOH) is a chemical property.
2. Saponification
Saponification is the process of soap making, where fats and oils (esters) react with a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH). This reaction breaks down the ester molecules into glycerol and fatty acid salts, which are the components of soap.
Example: The saponification of a triglyceride (a type of fat) with sodium hydroxide:
Triglyceride + 3NaOH → Glycerol + 3 Fatty Acid Sodium Salts
This reaction significantly alters the chemical composition of the reactants. The triglyceride, a complex ester, is converted into glycerol, a simpler alcohol, and fatty acid salts, which possess entirely different properties. The transformative nature of this reaction reinforces the classification of reactivity with a base (NaOH in this case) as a chemical property.
3. Reactions with Amphoteric Substances
Amphoteric substances can act as both acids and bases. Their reactivity with a base depends on the specific conditions and the strength of the base. For example, aluminum hydroxide, Al(OH)₃, is amphoteric. It can react with a strong base to form a complex ion:
Al(OH)₃(s) + OH⁻(aq) → [Al(OH)₄]⁻(aq)
This reaction involves the formation of a new complex ion, [Al(OH)₄]⁻, demonstrating a clear change in chemical composition and reaffirming that reactivity with a base is a chemical property.
4. Metal Oxide Reactions
Many metal oxides react with bases to form salts and water. These reactions are similar to neutralization reactions, but involve metal oxides instead of acids. For instance, aluminum oxide reacts with sodium hydroxide:
Al₂O₃(s) + 2NaOH(aq) + 3H₂O(l) → 2NaAl(OH)₄(aq)
The aluminum oxide reacts with the base to form sodium aluminate, a completely different chemical species. This transformation underscores the chemical nature of the reactivity.
Distinguishing Chemical from Physical Changes
It's crucial to distinguish between physical and chemical changes to properly understand reactivity. Physical changes alter the form or state of a substance without changing its chemical composition. Examples include melting, boiling, dissolving, and crushing. Chemical changes, on the other hand, lead to the formation of new substances with different chemical compositions. This involves the breaking and reforming of chemical bonds.
Reactivity with a base is always a chemical change because it involves the formation of new chemical compounds. The original substance and the base react to create new products with different properties. This is in stark contrast to physical changes, which only involve alterations in physical state or appearance.
Factors Affecting Base Reactivity
Several factors influence the reactivity of a substance with a base:
-
Strength of the base: Stronger bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH), tend to react more vigorously than weaker bases, such as ammonia (NH₃).
-
Nature of the substance: The chemical structure and properties of the substance significantly impact its reactivity. For example, some metals react readily with bases, while others do not.
-
Concentration of the base: Higher concentrations of base generally lead to faster reaction rates.
-
Temperature: Increasing temperature usually increases the rate of reaction.
Applications of Base Reactivity
The reactivity of substances with bases finds numerous applications across various fields:
-
Soap making (Saponification): As mentioned earlier, saponification relies on the reaction of fats and oils with bases to produce soap.
-
Industrial cleaning: Bases are used in various cleaning agents to dissolve grease, oils, and other substances.
-
Chemical synthesis: Base-catalyzed reactions are vital in the synthesis of many organic and inorganic compounds.
-
Water treatment: Bases are used to adjust the pH of water and to remove impurities.
-
Food processing: Bases play a role in several food processing applications, such as neutralizing acids and adjusting pH levels.
Conclusion: Reactivity with a Base – A Chemical Property
In conclusion, the reactivity of a substance with a base is undeniably a chemical property. The interaction always results in a chemical change, involving the breaking and forming of chemical bonds and the creation of new substances with different chemical compositions. Understanding this fundamental distinction between physical and chemical properties is essential for comprehending chemical reactions and their applications in various fields. The examples discussed – neutralization, saponification, reactions with amphoteric substances, and metal oxide reactions – all demonstrate the transformative nature of base reactivity, solidifying its classification as a crucial chemical property. Further exploration into the kinetics and thermodynamics of these reactions provides a deeper understanding of this important aspect of chemistry. The factors influencing reactivity, such as base strength, substance nature, concentration, and temperature, provide a framework for predicting and controlling the outcome of base-related reactions, making them a critical consideration in various applications from industrial processes to everyday life.
Latest Posts
Latest Posts
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Reactvity With A Base A Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
