Steps Of The Sliding Filament Theory
Muz Play
Mar 23, 2025 · 7 min read
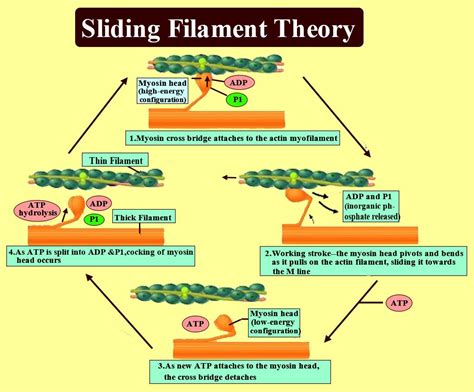
Table of Contents
Unveiling the Mechanics of Muscle Contraction: A Deep Dive into the Sliding Filament Theory
The human body is a marvel of engineering, capable of feats of strength, agility, and endurance. At the heart of this capability lies the intricate process of muscle contraction, a phenomenon explained by the sliding filament theory. This theory, a cornerstone of physiology, details the microscopic interactions that allow muscles to generate force and movement. Understanding its steps is crucial for comprehending not only how our bodies function but also for advancements in fields like sports medicine, rehabilitation, and the development of therapeutic interventions for muscle disorders. This comprehensive guide will meticulously explore each step of the sliding filament theory, providing a detailed, yet accessible, explanation for both students and enthusiasts alike.
Step 1: The Arrival of the Action Potential
The story begins with a nerve impulse, or action potential, arriving at the neuromuscular junction – the specialized synapse where a motor neuron interacts with a muscle fiber. This action potential triggers the release of acetylcholine, a neurotransmitter, into the synaptic cleft. Acetylcholine diffuses across the cleft and binds to receptors on the muscle fiber's sarcolemma (cell membrane).
Initiating the Cascade: Depolarization and Calcium Release
The binding of acetylcholine initiates a cascade of events leading to muscle fiber depolarization. This depolarization spreads along the sarcolemma and down the T-tubules, invaginations of the sarcolemma that penetrate deep into the muscle fiber. The T-tubules are crucial for transmitting the electrical signal to the sarcoplasmic reticulum (SR), a specialized intracellular organelle that stores calcium ions (Ca²⁺).
The depolarization signal reaching the T-tubules triggers the release of Ca²⁺ from the SR into the sarcoplasm, the cytoplasm of the muscle fiber. This sudden increase in intracellular Ca²⁺ concentration is the critical trigger for muscle contraction. This calcium release is highly regulated, ensuring precise control over muscle activity. Any disruption to this process can result in muscle weakness or dysfunction.
Step 2: Calcium Binding to Troponin
The released Ca²⁺ ions bind to a protein complex called troponin, located on the thin filaments of the sarcomere – the basic contractile unit of a muscle fiber. Troponin is composed of three subunits: troponin I (TnI), troponin T (TnT), and troponin C (TnC). TnC has high affinity for Ca²⁺ and its binding initiates the conformational change that initiates contraction.
Unmasking the Binding Sites: The Role of Tropomyosin
Prior to Ca²⁺ binding, another protein, tropomyosin, blocks the myosin-binding sites on the actin filaments. Tropomyosin acts like a physical barrier, preventing the interaction between actin and myosin, the proteins responsible for muscle contraction. The Ca²⁺-troponin interaction causes a conformational change in tropomyosin, moving it away from the myosin-binding sites on actin, making them accessible to myosin. This "unmasking" of the binding sites is a pivotal step, allowing the interaction that drives muscle contraction.
Step 3: Cross-Bridge Formation and Power Stroke
With the myosin-binding sites exposed, the myosin heads, projecting from the thick filaments, can now bind to actin. This interaction forms a cross-bridge. The myosin heads are in a high-energy state due to the hydrolysis of ATP (adenosine triphosphate) – the energy currency of the cell.
The Power Stroke: Sliding Filaments
The binding of myosin to actin triggers a conformational change in the myosin head, causing it to pivot and exert a force – the power stroke. This movement pulls the thin filaments towards the center of the sarcomere, causing the sarcomere to shorten. This shortening of individual sarcomeres collectively leads to the overall contraction of the muscle fiber. The power stroke is an energy-dependent process, requiring the hydrolysis of ATP to fuel the conformational change in the myosin head.
Step 4: Cross-Bridge Detachment and ATP Hydrolysis
After the power stroke, the myosin head remains bound to the actin filament. To initiate another cycle, the myosin head must detach. This detachment requires another ATP molecule. The binding of ATP to the myosin head causes a conformational change, reducing its affinity for actin and leading to cross-bridge detachment.
The ATPase Activity of Myosin: Fueling the Cycle
The ATP molecule is then hydrolyzed by the ATPase activity of the myosin head. This hydrolysis regenerates the high-energy state of the myosin head, preparing it for another cycle of cross-bridge formation, power stroke, and detachment. The continuous cycle of cross-bridge formation, power stroke, and detachment is what drives the sustained contraction of the muscle fiber. The efficiency of ATP hydrolysis is crucial for the muscle's ability to generate force.
Step 5: Calcium Removal and Relaxation
For muscle relaxation to occur, the intracellular Ca²⁺ concentration must be reduced. The SR actively pumps Ca²⁺ back into its lumen, lowering the cytosolic Ca²⁺ concentration. As the Ca²⁺ levels decrease, it detaches from troponin.
Returning to Resting State: Tropomyosin and Muscle Relaxation
This detachment triggers a conformational change in troponin, which in turn restores tropomyosin's position, blocking the myosin-binding sites on actin. With the binding sites blocked, cross-bridge formation is prevented, and muscle contraction ceases. The muscle fiber returns to its resting length. This regulated calcium removal is essential for preventing continuous muscle contraction and allowing for controlled movement. Disruptions in this process can lead to muscle rigidity or spasms.
Factors Influencing Muscle Contraction: Beyond the Basics
The sliding filament theory provides a fundamental framework for understanding muscle contraction, but several other factors contribute to the complexity and fine-tuning of this process.
The Role of Neural Input: Frequency and Strength of Stimulation
The frequency and strength of the neural stimulation significantly influence muscle contraction. High-frequency stimulation can lead to tetanus, a sustained contraction where individual muscle twitches fuse together. Conversely, low-frequency stimulation results in a series of distinct twitches. The strength of contraction is also dependent on the number of motor units recruited.
Energy Supply: The Importance of ATP
The availability of ATP is crucial for muscle contraction. During intense activity, ATP stores can be depleted, leading to muscle fatigue. Different metabolic pathways provide ATP, ranging from aerobic respiration (using oxygen) to anaerobic glycolysis (without oxygen). The choice of metabolic pathway depends on the intensity and duration of the muscular activity.
Muscle Fiber Types: Variations in Contractile Properties
Different types of muscle fibers have varying contractile properties, reflecting adaptations to different functional demands. Slow-twitch fibers are resistant to fatigue but generate relatively low force, while fast-twitch fibers generate high force but fatigue more quickly. The proportion of different fiber types in a muscle determines its overall performance characteristics.
Muscle Length and Tension: The Length-Tension Relationship
The length of a muscle fiber at the onset of contraction affects the force it can generate. There is an optimal length where the overlap between actin and myosin is maximized, resulting in maximal force production. Shorter or longer lengths result in reduced force generation. This length-tension relationship is vital for optimizing muscle performance.
Clinical Significance and Future Directions
Understanding the sliding filament theory has profound implications for various clinical applications. Conditions like muscular dystrophy, myasthenia gravis, and various types of muscle injuries often involve disruptions in the steps of the sliding filament process. Research focusing on these disruptions is critical for developing effective diagnostic tools and therapeutic strategies.
Therapeutic Interventions: Targeting the Molecular Machinery
Future research will likely focus on developing targeted therapies that address specific molecular components of the contractile machinery. This may include drugs that enhance calcium handling, modulate myosin ATPase activity, or improve the efficiency of energy metabolism. Such advancements could significantly improve the lives of individuals suffering from muscle disorders.
Understanding Aging and Muscle Function: Preserving Strength and Mobility
Aging is associated with a decline in muscle mass and function, a process known as sarcopenia. Understanding the molecular mechanisms underlying age-related muscle loss is crucial for developing interventions to preserve muscle strength and mobility in older adults. This understanding will rely on a deeper understanding of the sliding filament process and its age-related alterations.
In conclusion, the sliding filament theory provides a comprehensive explanation of the intricate process of muscle contraction. Understanding its steps is paramount for appreciating the remarkable capabilities of the human body and for developing effective therapeutic strategies for muscle-related disorders. Ongoing research continues to refine our understanding of this fundamental biological process, promising exciting advancements in the years to come. From sports performance to geriatric care, the sliding filament theory holds the key to unlocking significant improvements in health and well-being.
Latest Posts
Latest Posts
-
Electric Field Between Two Opposite Charges
Mar 25, 2025
-
In A Molecule With Covalent Bonding
Mar 25, 2025
-
Atoms That Gain Or Lose Electrons Are Known As
Mar 25, 2025
-
Programmed Decision And Non Programmed Decision
Mar 25, 2025
-
Electric Field Of Uniformly Charged Disk
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Steps Of The Sliding Filament Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
