Substitution And Elimination Organic Chemistry Practice Problems
Muz Play
Mar 31, 2025 · 5 min read
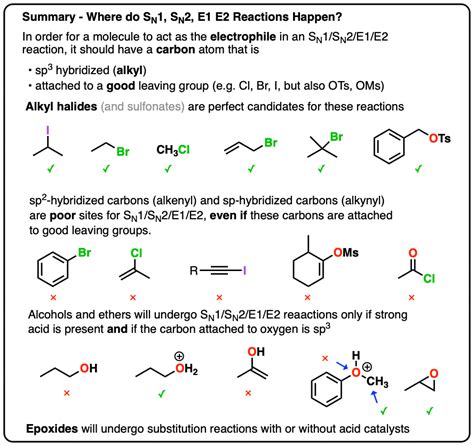
Table of Contents
Substitution and Elimination Reactions: Mastering Organic Chemistry Through Practice Problems
Organic chemistry, often considered a daunting subject, hinges heavily on understanding reaction mechanisms. Among the most fundamental are substitution and elimination reactions, which involve the replacement or removal of atoms or groups from a molecule. Mastering these requires a solid grasp of concepts and ample practice. This comprehensive guide provides numerous practice problems, ranging from basic to advanced, to solidify your understanding of substitution and elimination reactions.
Understanding the Fundamentals: SN1, SN2, E1, and E2 Reactions
Before diving into the problems, let's briefly review the four primary reaction types:
1. SN1 (Substitution Nucleophilic Unimolecular):
- Mechanism: Two-step process. The first step involves the departure of the leaving group, forming a carbocation intermediate. The second step involves the nucleophile attacking the carbocation.
- Rate Determining Step: The formation of the carbocation (first step). The rate is dependent only on the concentration of the substrate (rate = k[substrate]).
- Stereochemistry: Racemization occurs due to the planar nature of the carbocation intermediate.
- Favored by: Tertiary substrates, weak nucleophiles, polar protic solvents.
2. SN2 (Substitution Nucleophilic Bimolecular):
- Mechanism: One-step concerted mechanism. The nucleophile attacks the substrate from the backside, simultaneously displacing the leaving group.
- Rate Determining Step: The single step involving both the nucleophile and substrate. The rate is dependent on the concentration of both the substrate and the nucleophile (rate = k[substrate][nucleophile]).
- Stereochemistry: Inversion of configuration occurs at the chiral center.
- Favored by: Primary substrates, strong nucleophiles, polar aprotic solvents.
3. E1 (Elimination Unimolecular):
- Mechanism: Two-step process. The first step involves the departure of the leaving group, forming a carbocation intermediate. The second step involves the removal of a proton from a carbon adjacent to the carbocation by a base, forming a double bond.
- Rate Determining Step: The formation of the carbocation (first step). The rate is dependent only on the concentration of the substrate (rate = k[substrate]).
- Favored by: Tertiary substrates, weak bases, polar protic solvents. Often competes with SN1.
- Product Distribution: Often leads to a mixture of Zaitsev and Hofmann products (Zaitsev being the more substituted alkene, Hofmann being the less substituted).
4. E2 (Elimination Bimolecular):
- Mechanism: One-step concerted mechanism. The base abstracts a proton from a carbon adjacent to the leaving group, while simultaneously the leaving group departs, forming a double bond.
- Rate Determining Step: The single step involving both the substrate and the base. The rate is dependent on the concentration of both the substrate and the base (rate = k[substrate][base]).
- Favored by: Strong bases, primary and secondary substrates. Often competes with SN2.
- Stereochemistry: Requires anti-periplanar geometry between the leaving group and the proton being abstracted.
- Product Distribution: Primarily forms the Zaitsev product.
Practice Problems: Substitution and Elimination Reactions
Let's move on to the practice problems. Remember to consider the substrate structure, nucleophile/base strength, solvent, and steric hindrance when predicting the outcome.
Problem 1: Predict the major product(s) of the reaction between 2-bromobutane and sodium methoxide (NaOCH₃) in methanol.
Problem 2: Predict the major product(s) of the reaction between tert-butyl bromide and potassium hydroxide (KOH) in ethanol.
Problem 3: What is the major product formed when 1-chloropropane reacts with sodium iodide (NaI) in acetone?
Problem 4: Predict the major product of the reaction between 2-chloro-2-methylpropane and sodium hydroxide (NaOH) in water. Will this reaction proceed via an SN1, SN2, E1, or E2 mechanism? Justify your answer.
Problem 5: Which of the following substrates will undergo SN1 reaction faster: 2-bromopropane or 2-bromo-2-methylpropane? Explain your reasoning.
Problem 6: Draw the mechanism for the SN2 reaction between bromomethane and sodium cyanide (NaCN). Show all the electron movements using curved arrows.
Problem 7: Draw the mechanism for the E1 reaction of 2-bromo-2-methylpropane in the presence of ethanol.
Problem 8: Explain why SN2 reactions are favored by strong nucleophiles and polar aprotic solvents.
Problem 9 (Advanced): Predict the major product of the reaction between (R)-2-bromobutane and sodium ethoxide in ethanol. Indicate the stereochemistry of the product.
Problem 10 (Advanced): A chiral alkyl halide undergoes solvolysis in water to yield a racemic mixture of alcohols. What mechanism is most likely responsible? Explain your answer.
Solutions and Explanations
Problem 1: This reaction will proceed via an SN2 mechanism due to the primary nature of the substrate and the strong nucleophile (methoxide). The major product will be 2-methoxybutane.
Problem 2: This reaction favors an E2 mechanism due to the tertiary substrate and strong base. The major product will be 2-methylpropene (isobutylene), the Zaitsev product.
Problem 3: This reaction is an SN2 reaction, leading to the formation of 1-iodopropane.
Problem 4: This reaction will proceed via an E2 mechanism due to the strong base and tertiary substrate. The major product will be 2-methylpropene.
Problem 5: 2-bromo-2-methylpropane will undergo SN1 reaction faster. This is because the tertiary carbocation formed is more stable than the secondary carbocation formed from 2-bromopropane.
Problem 6: (This requires a diagram showing the nucleophile attacking the carbon atom bonded to the bromine, with the bromine leaving; curved arrows show electron movement.)
Problem 7: (This requires a diagram showing the formation of a carbocation intermediate followed by proton abstraction leading to the alkene product.)
Problem 8: Strong nucleophiles are favored because they readily attack the substrate. Polar aprotic solvents solvate the cations but leave the nucleophile relatively unhindered, increasing its nucleophilicity.
Problem 9: This reaction will proceed via an SN2 mechanism, resulting in inversion of configuration. The product will be (S)-2-ethoxybutane.
Problem 10: An SN1 mechanism is most likely responsible because the formation of a planar carbocation intermediate allows for attack by the nucleophile from either side, leading to a racemic mixture.
Further Practice and Resources
These problems provide a foundation for understanding substitution and elimination reactions. For more in-depth practice, consider working through problems in your textbook or seeking out online resources. Remember that consistent practice and a thorough understanding of the reaction mechanisms are key to mastering this crucial aspect of organic chemistry. Focus on understanding why certain reactions favor specific mechanisms and product distributions. This deep understanding, combined with diligent practice, will ultimately enhance your problem-solving skills and significantly improve your grasp of organic chemistry. Don't be afraid to revisit these fundamentals and practice consistently; mastery takes time and effort.
Latest Posts
Latest Posts
-
Name The Four Social Change Theories
Apr 02, 2025
-
Which Of These Infectious Agents Do Not Have Nucleic Acid
Apr 02, 2025
-
How Many Total Atp Are Produced During Glycolysis
Apr 02, 2025
-
Mean And Standard Deviation Of Sampling Distribution Calculator
Apr 02, 2025
-
Dorsal View Of The Sheep Brain
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Substitution And Elimination Organic Chemistry Practice Problems . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
