Temperature Measure Of Average Molecular Translational Kinestic Energty
Muz Play
Mar 16, 2025 · 5 min read
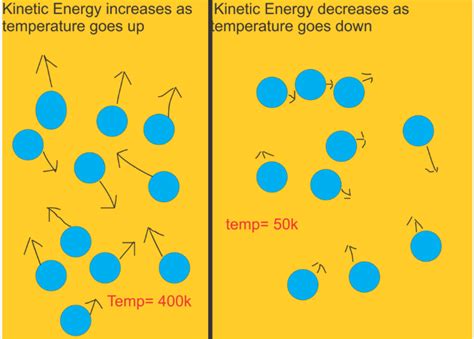
Table of Contents
- Temperature Measure Of Average Molecular Translational Kinestic Energty
- Table of Contents
- Temperature: A Measure of Average Molecular Translational Kinetic Energy
- The Microscopic View: Kinetic Energy and Molecular Motion
- Defining Translational Kinetic Energy
- Connecting the Microscopic and Macroscopic: Temperature as an Average
- Absolute Zero: The Cessation of Motion
- The Ideal Gas Law and Kinetic Energy
- Beyond Ideal Gases: Real-World Applications
- Real Gases and Intermolecular Forces
- Liquids and Solids: More Complex Motion
- Applications in Various Fields
- Measuring Temperature: Different Methods and Their Relation to Kinetic Energy
- Expansion of Matter
- Electrical Resistance
- Thermocouples
- Infrared Thermography
- Conclusion: A Fundamental Link
- Latest Posts
- Latest Posts
- Related Post
Temperature: A Measure of Average Molecular Translational Kinetic Energy
Temperature, a fundamental concept in physics and chemistry, is much more than just a number on a thermometer. It's a direct measure of the average translational kinetic energy of the particles (atoms or molecules) within a substance. Understanding this connection is crucial to grasping the behavior of matter at different scales, from individual molecules to macroscopic systems. This article will delve deep into the relationship between temperature and molecular kinetic energy, exploring its implications across various thermodynamic concepts.
The Microscopic View: Kinetic Energy and Molecular Motion
At the microscopic level, matter is composed of countless particles in constant, chaotic motion. This motion isn't just vibrational or rotational; a significant part is translational kinetic energy, the energy associated with movement from one location to another. Imagine a gas: its molecules are zipping around in all directions, colliding with each other and the container walls. The faster they move, the higher their individual kinetic energies.
Defining Translational Kinetic Energy
The translational kinetic energy (KE) of a single molecule is given by the classic formula:
KE = ½mv²
Where:
- m represents the mass of the molecule.
- v represents the speed of the molecule.
It's crucial to note that molecules in a substance don't all possess the same speed. Their speeds follow a distribution, often described by the Maxwell-Boltzmann distribution. This distribution shows that while some molecules move incredibly fast, others move much slower. The distribution's shape depends on the temperature and mass of the molecules.
Connecting the Microscopic and Macroscopic: Temperature as an Average
Temperature, a macroscopic property, is directly linked to the average translational kinetic energy of the molecules. A higher temperature signifies a greater average kinetic energy. This means the molecules, on average, are moving faster. Conversely, a lower temperature indicates slower average molecular motion.
Absolute Zero: The Cessation of Motion
The concept of temperature finds its theoretical limit at absolute zero (0 Kelvin or -273.15°C). At this point, all molecular motion, and hence translational kinetic energy, theoretically ceases. However, reaching absolute zero is practically impossible due to quantum mechanical effects.
The Ideal Gas Law and Kinetic Energy
The ideal gas law, PV = nRT, provides a macroscopic connection to molecular kinetic energy. While seemingly unrelated to individual molecule speeds, the ideal gas law implicitly incorporates the average kinetic energy. The constant R (the ideal gas constant) links macroscopic pressure and volume to the microscopic energy of the gas molecules.
A more direct connection is shown through the following derivation:
The average kinetic energy of an ideal gas molecule can be expressed as:
<KE> = (3/2)kT
Where:
- <KE> represents the average translational kinetic energy.
- k represents the Boltzmann constant (1.38 x 10⁻²³ J/K).
- T represents the absolute temperature in Kelvin.
This equation beautifully illustrates the direct proportionality between temperature and average kinetic energy. A doubling of the absolute temperature results in a doubling of the average kinetic energy.
Beyond Ideal Gases: Real-World Applications
While the ideal gas model simplifies reality by neglecting intermolecular forces and molecular volume, the fundamental relationship between temperature and average kinetic energy still holds true, albeit with modifications, for real gases and even liquids and solids.
Real Gases and Intermolecular Forces
Real gases deviate from ideal behavior, particularly at high pressures and low temperatures. Intermolecular forces, such as van der Waals forces, become significant, affecting the average kinetic energy and influencing the pressure-volume relationship. However, the core principle—temperature reflecting the average kinetic energy—remains valid.
Liquids and Solids: More Complex Motion
In liquids and solids, molecular motion is more constrained than in gases. While translational kinetic energy still contributes, vibrational and rotational energies become increasingly important. Nevertheless, temperature still acts as an indicator of the average total kinetic energy, encompassing all forms of molecular motion. As temperature increases, the amplitude of vibrational and rotational motions increases, leading to increased average kinetic energy.
Applications in Various Fields
Understanding the link between temperature and average molecular kinetic energy has profound implications across numerous scientific and engineering disciplines:
- Thermodynamics: The concept is fundamental in defining thermodynamic properties like internal energy, enthalpy, and entropy.
- Chemical Kinetics: Reaction rates are directly influenced by the average kinetic energy of reactant molecules. Higher temperatures lead to faster reactions due to increased collision frequency and energy.
- Material Science: The properties of materials (strength, conductivity, etc.) are strongly influenced by the kinetic energy of their constituent particles.
- Meteorology: Temperature measurements are crucial in understanding atmospheric processes, weather patterns, and climate change.
- Astronomy: Studying the temperature of stars and other celestial bodies provides insights into their composition and evolution.
Measuring Temperature: Different Methods and Their Relation to Kinetic Energy
Various methods exist for measuring temperature, each exploiting different physical properties sensitive to average molecular kinetic energy.
Expansion of Matter
Thermometers based on the expansion of liquids (like mercury or alcohol) rely on the principle that increased temperature leads to increased molecular kinetic energy, causing the substance to expand. The expansion is directly calibrated to a temperature scale.
Electrical Resistance
Thermistors and resistance temperature detectors (RTDs) utilize the change in electrical resistance of a material with temperature. This change is directly related to changes in the average kinetic energy of the electrons within the material.
Thermocouples
Thermocouples, based on the Seebeck effect, generate a voltage proportional to the temperature difference between two dissimilar metals. This voltage arises from the movement of charge carriers, which is again intrinsically linked to the average kinetic energy of the electrons.
Infrared Thermography
Infrared thermography measures the emitted infrared radiation from an object. The intensity of this radiation is directly related to the temperature and therefore to the average kinetic energy of the molecules within the object.
Conclusion: A Fundamental Link
The relationship between temperature and average molecular translational kinetic energy is a cornerstone of our understanding of the physical world. While the ideal gas model provides a simplified representation, the fundamental principle applies across all phases of matter, albeit with increased complexity. By understanding this relationship, we can effectively interpret and predict the behaviour of matter on both microscopic and macroscopic scales, paving the way for advancement in countless scientific and engineering fields. This connection underscores the power of linking the microscopic world of atoms and molecules with the macroscopic world we observe and experience.
Latest Posts
Latest Posts
-
A Chemical Combination Of Two Or More Elements Is A
Mar 16, 2025
-
Does Slt Water Break Down Rocks Or Compact Them
Mar 16, 2025
-
An Organism That Cannot Grow Without Oxygen Is A
Mar 16, 2025
-
Electron Configuration For A Neutral Atom Of Phosphorus
Mar 16, 2025
-
Mitosis Of An Onion Root Tip
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Temperature Measure Of Average Molecular Translational Kinestic Energty . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
