The Arrangement Of Electrons In An Atom
Muz Play
Mar 18, 2025 · 6 min read
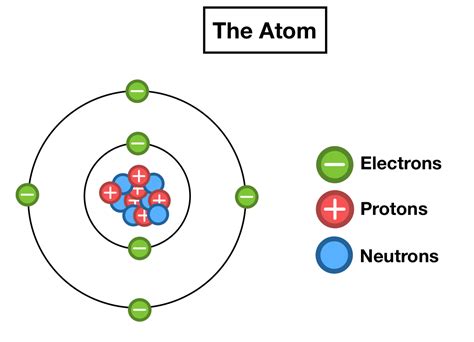
Table of Contents
The Arrangement of Electrons in an Atom: Unveiling the Secrets of Atomic Structure
The atom, the fundamental building block of matter, is a fascinating world of subatomic particles. While protons and neutrons reside in the atom's nucleus, electrons occupy a much more dynamic and complex realm – the electron cloud. Understanding the arrangement of these electrons is crucial to comprehending the properties of elements, their interactions, and the formation of molecules. This article delves into the intricacies of electron arrangement, exploring key concepts like electron shells, subshells, orbitals, and the principles governing their organization.
Electron Shells and Energy Levels
Electrons don't randomly float around the nucleus. Instead, they occupy specific energy levels, often visualized as concentric shells surrounding the nucleus. These shells represent distinct regions of space where electrons are most likely to be found. The closer a shell is to the nucleus, the lower its energy level. Electrons in shells closer to the nucleus experience a stronger attractive force from the positively charged protons, resulting in lower energy.
Principal Quantum Number (n)
The principal quantum number, n, designates the electron shell and its energy level. n can take on positive integer values (1, 2, 3, and so on). n = 1 represents the shell closest to the nucleus (the K shell), n = 2 represents the next shell (the L shell), and so on. The higher the value of n, the farther the shell is from the nucleus and the higher its energy.
Shell Capacity
Each electron shell has a maximum number of electrons it can hold. This capacity is determined by the formula 2n<sup>2</sup>. Therefore:
- n = 1 (K shell): 2(1)<sup>2</sup> = 2 electrons
- n = 2 (L shell): 2(2)<sup>2</sup> = 8 electrons
- n = 3 (M shell): 2(3)<sup>2</sup> = 18 electrons
- n = 4 (N shell): 2(4)<sup>2</sup> = 32 electrons
and so on.
Subshells and Orbital Shapes
While the shell model provides a basic understanding, it's an oversimplification. Within each shell, electrons are further organized into subshells, each characterized by a specific shape and energy level.
Azimuthal Quantum Number (l)
The azimuthal quantum number, l, defines the subshell within a shell. For a given value of n, l can range from 0 to n - 1. Each value of l corresponds to a different subshell:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Each subshell can hold a specific number of electrons:
- s subshell: 2 electrons
- p subshell: 6 electrons
- d subshell: 10 electrons
- f subshell: 14 electrons
Orbitals and Electron Spin
Subshells are further divided into orbitals. An orbital is a region of space within a subshell where there's a high probability of finding an electron.
Magnetic Quantum Number (ml)
The magnetic quantum number, m<sub>l</sub>, specifies the orientation of an orbital in space. For a given value of l, m<sub>l</sub> can range from -l to +l, including 0. This means:
- s subshell (l=0): 1 orbital
- p subshell (l=1): 3 orbitals (px, py, pz)
- d subshell (l=2): 5 orbitals
- f subshell (l=3): 7 orbitals
Each orbital can hold a maximum of two electrons.
Spin Quantum Number (ms)
The spin quantum number, m<sub>s</sub>, describes the intrinsic angular momentum of an electron, often referred to as its "spin." Each electron can have one of two spin states: +1/2 (spin up) or -1/2 (spin down). The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, m<sub>l</sub>, m<sub>s</sub>). This principle dictates that each orbital can hold a maximum of two electrons, with opposite spins.
Electron Configuration and Filling Order
The electron configuration of an atom describes the arrangement of electrons in its shells, subshells, and orbitals. Electrons fill orbitals in a specific order, following the Aufbau principle (building-up principle), Hund's rule, and the Pauli exclusion principle.
Aufbau Principle
The Aufbau principle states that electrons first fill the lowest energy levels available. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… Note that there are exceptions to this order, particularly for transition metals and lanthanides/actinides.
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before pairing up. This minimizes electron-electron repulsion. For example, in a p subshell with three orbitals, the three electrons will each occupy a separate orbital with parallel spins before any pairing occurs.
Writing Electron Configurations
Electron configurations are written using a shorthand notation. For example, the electron configuration of oxygen (atomic number 8) is 1s<sup>2</sup>2s<sup>2</sup>2p<sup>4</sup>. This means there are two electrons in the 1s orbital, two in the 2s orbital, and four in the 2p orbitals.
Valence Electrons and Chemical Bonding
The electrons in the outermost shell of an atom are called valence electrons. These electrons are crucial in determining an element's chemical properties and its ability to form chemical bonds with other atoms. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (a full outermost shell). This drive towards stability underlies the formation of ionic and covalent bonds.
Ionic Bonding
In ionic bonding, atoms transfer electrons to achieve a stable electron configuration. One atom loses electrons (becoming a positively charged cation) while another atom gains electrons (becoming a negatively charged anion). The electrostatic attraction between the oppositely charged ions forms the ionic bond.
Covalent Bonding
In covalent bonding, atoms share electrons to achieve stable electron configurations. This sharing creates a bond between the atoms, resulting in the formation of molecules.
Exceptions to the Rules
While the Aufbau principle, Hund's rule, and the Pauli exclusion principle generally govern electron configurations, there are exceptions, particularly for transition metals and lanthanides/actinides. These exceptions often arise due to the subtle energy differences between orbitals and the complex interactions between electrons. Understanding these exceptions requires a deeper dive into advanced quantum mechanics.
Advanced Concepts and Applications
The arrangement of electrons in an atom is a fundamental concept with far-reaching applications. It plays a critical role in various fields, including:
-
Chemistry: Understanding electron configurations is essential for predicting the reactivity of elements, explaining chemical bonding, and understanding the properties of molecules.
-
Materials Science: The arrangement of electrons determines the electrical conductivity, magnetic properties, and other physical properties of materials. This knowledge is crucial for designing and developing new materials with desired characteristics.
-
Spectroscopy: Analyzing the light emitted or absorbed by atoms provides information about their electron configurations and energy levels. Spectroscopy is a powerful tool used in various scientific disciplines, including astronomy and analytical chemistry.
-
Nuclear Physics: The stability of atomic nuclei is influenced by the arrangement of electrons, and understanding this relationship is important in nuclear physics research.
Conclusion
The arrangement of electrons in an atom is a complex yet fascinating topic that underpins much of our understanding of chemistry, physics, and materials science. While the basic principles are relatively straightforward, a deeper appreciation requires exploring the nuances of quantum mechanics and understanding the exceptions to the rules. Mastering these concepts opens doors to a deeper understanding of the world around us, from the smallest atoms to the largest stars. The seemingly simple arrangement of electrons holds the key to unlocking countless scientific discoveries and technological advancements.
Latest Posts
Latest Posts
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Arrangement Of Electrons In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
