The Higher The Boiling Point The More Polar
Muz Play
Mar 22, 2025 · 5 min read
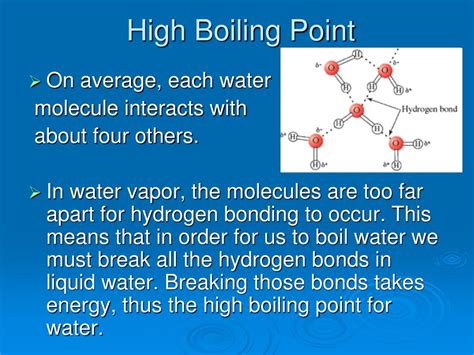
Table of Contents
The Higher the Boiling Point, the More Polar: Understanding the Relationship Between Boiling Point and Polarity
The relationship between boiling point and polarity is a fundamental concept in chemistry. While not always a strictly linear correlation, a higher boiling point generally indicates stronger intermolecular forces, which are often associated with greater polarity. This article delves into the intricacies of this relationship, exploring the various factors that influence boiling point and how polarity plays a crucial role. We'll examine different types of intermolecular forces, providing examples and explaining the underlying principles that govern the behavior of molecules.
Understanding Boiling Point
Boiling point is the temperature at which a liquid transitions to a gas phase. This transition occurs when the kinetic energy of the molecules overcomes the intermolecular forces holding them together in the liquid state. The stronger the intermolecular forces, the more energy (and thus, higher temperature) is required to overcome them, resulting in a higher boiling point.
Factors Affecting Boiling Point
Several factors influence a substance's boiling point, including:
-
Molecular Weight: Heavier molecules generally have higher boiling points because they possess stronger London Dispersion Forces (LDFs), a type of intermolecular force discussed in detail below. Larger molecules have more electrons, leading to stronger temporary dipoles and greater attraction.
-
Molecular Shape: The shape of a molecule influences the extent to which intermolecular forces can operate. Linear molecules tend to have higher boiling points than branched molecules of similar molecular weight because they pack more efficiently, leading to stronger interactions.
-
Intermolecular Forces: This is the most significant factor determining boiling point. The strength of the intermolecular forces directly correlates with the energy required to separate the molecules, hence impacting the boiling point.
The Role of Polarity in Intermolecular Forces
Polarity arises from the uneven distribution of electrons within a molecule. This uneven distribution creates a dipole moment, where one end of the molecule has a slightly positive charge (δ+) and the other end has a slightly negative charge (δ-). The presence of polar bonds and the overall molecular geometry determine the molecule's polarity.
Types of Intermolecular Forces
Understanding the different types of intermolecular forces is crucial to grasping the connection between boiling point and polarity:
-
London Dispersion Forces (LDFs): These are the weakest type of intermolecular forces and are present in all molecules. They arise from temporary, instantaneous dipoles created by the random movement of electrons. Larger molecules with more electrons exhibit stronger LDFs due to increased polarizability.
-
Dipole-Dipole Forces: These forces occur between polar molecules. The positive end of one polar molecule attracts the negative end of another, leading to a stronger interaction than LDFs. The strength of dipole-dipole forces increases with the magnitude of the dipole moment.
-
Hydrogen Bonding: This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine). Hydrogen bonds are significantly stronger than typical dipole-dipole forces and result in substantially higher boiling points for molecules capable of forming them.
Polarity and Boiling Point: A Closer Look
The strength of intermolecular forces directly impacts boiling point. Polar molecules, due to their dipole moments, experience stronger intermolecular forces (dipole-dipole forces and potentially hydrogen bonding) compared to nonpolar molecules which only exhibit LDFs. Therefore, polar molecules generally have higher boiling points than nonpolar molecules of similar molecular weight.
Examples Illustrating the Relationship
Let's compare some examples to illustrate this principle:
-
Water (H₂O) vs. Methane (CH₄): Water, a highly polar molecule with strong hydrogen bonding, has a boiling point of 100°C. Methane, a nonpolar molecule with only LDFs, has a boiling point of -161.5°C. The vast difference in boiling points highlights the significant impact of polarity and hydrogen bonding.
-
Ethanol (C₂H₅OH) vs. Dimethyl ether (CH₃OCH₃): Both molecules have the same molecular formula (C₂H₆O), but their structures differ significantly. Ethanol is polar due to the presence of an –OH group capable of hydrogen bonding, resulting in a boiling point of 78.4°C. Dimethyl ether, which is less polar and cannot form hydrogen bonds, has a boiling point of -23.6°C.
-
Acetone (CH₃COCH₃) vs. Propane (C₃H₈): Acetone, a polar molecule with dipole-dipole forces, boils at 56.05°C. Propane, a nonpolar molecule relying solely on LDFs, boils at -42°C. Again, the difference in boiling point demonstrates the effect of polarity.
Exceptions and Nuances
While the general trend is that higher polarity leads to higher boiling points, there are exceptions and nuances to consider:
-
Molecular Size: For molecules with significantly different molecular weights, the impact of LDFs can outweigh the influence of polarity. A very large nonpolar molecule might have a higher boiling point than a smaller polar molecule.
-
Branching: Branched molecules have lower boiling points than their linear counterparts due to reduced surface area for intermolecular interactions, even if they are polar.
Conclusion: The Importance of Understanding the Relationship
The relationship between boiling point and polarity is a crucial aspect of understanding the physical properties of molecules. While not a universally absolute rule, the general principle holds true: stronger intermolecular forces, often associated with higher polarity, result in higher boiling points. Understanding this relationship is essential in various fields, including chemistry, materials science, and pharmacology, allowing scientists and engineers to predict and manipulate the properties of substances. By considering factors such as molecular weight, shape, and the presence of various intermolecular forces, we can gain a comprehensive understanding of how polarity affects boiling points and other physical properties of matter. This knowledge is fundamental for many applications, including designing new materials with specific boiling points or understanding the behavior of molecules in different environments. The interplay of polarity and boiling point is a complex but fascinating area of study that continues to yield insights into the nature of matter.
Latest Posts
Latest Posts
-
Interphase In An Onion Root Tip
Mar 23, 2025
-
Addition And Subtraction Properties Of Equality
Mar 23, 2025
-
How To Calculate Kb When Only Given Ka
Mar 23, 2025
-
Steps For Conducting A Presumptive Blood Test
Mar 23, 2025
-
The Plasma Membrane Of A Muscle Fiber Is Called The
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about The Higher The Boiling Point The More Polar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
