The Movement Of Water Across A Semipermeable Membrane Is Called
Muz Play
Mar 18, 2025 · 6 min read
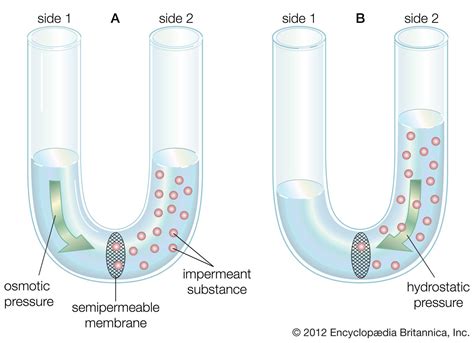
Table of Contents
The Movement of Water Across a Semipermeable Membrane is Called Osmosis: A Deep Dive
The movement of water across a semipermeable membrane is called osmosis. This seemingly simple process is fundamental to life itself, impacting everything from the turgidity of plants to the function of our kidneys. Understanding osmosis requires delving into the concepts of semipermeable membranes, concentration gradients, osmotic pressure, and the various types of osmotic solutions. This comprehensive guide will explore these concepts, explaining their significance in biological systems and beyond.
What is a Semipermeable Membrane?
Before diving into osmosis, it's crucial to understand what a semipermeable membrane is. A semipermeable membrane (also known as a selectively permeable membrane or differentially permeable membrane) is a type of biological or synthetic membrane that allows certain molecules or ions to pass through it by diffusion, while restricting the passage of others. This selective permeability is key to osmosis. The membrane's structure dictates which molecules can pass and which cannot.
Think of it like a sieve: small particles can pass through the holes, while larger ones are blocked. In biological systems, these membranes are often composed of lipids, proteins, and carbohydrates, forming a complex structure with specific channels and pores that control the passage of substances. The size, charge, and polarity of the molecules all influence their ability to cross the membrane.
Examples of Semipermeable Membranes:
- Cell membranes (plasma membranes): These membranes surround all living cells, regulating the passage of water, nutrients, and waste products. They are crucial for maintaining cellular homeostasis.
- Vacuolar membranes (tonoplasts): These membranes enclose vacuoles in plant cells, controlling the movement of water and other substances into and out of these storage compartments.
- Dialysis tubing: This artificial membrane is often used in experiments to demonstrate osmosis. It has pores of a specific size that allow certain molecules to pass through.
Understanding Osmosis: The Movement of Water
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. It's important to note that osmosis is driven by the difference in water potential, not by the pressure differences across the membrane.
Water potential is the measure of the free energy of water. It's influenced by factors like solute concentration and pressure. Water moves from an area of higher water potential to an area of lower water potential. A higher solute concentration leads to a lower water potential.
Key Concepts Related to Osmosis:
- Solute: A substance dissolved in a solvent to form a solution.
- Solvent: The substance that dissolves a solute to form a solution (in osmosis, the solvent is usually water).
- Solution: A homogeneous mixture of two or more substances.
- Concentration gradient: The difference in concentration of a substance between two areas.
Osmotic Pressure: The Driving Force
Osmotic pressure is the pressure that needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane. It's a measure of the tendency of a solution to draw water into itself by osmosis. The higher the solute concentration, the higher the osmotic pressure.
Imagine a solution separated from pure water by a semipermeable membrane. Water will move from the pure water (high water potential) into the solution (low water potential). The pressure exerted by the water entering the solution is the osmotic pressure. If you were to apply an equal and opposite pressure to the solution, you could prevent further water movement.
Types of Osmotic Solutions:
Based on the relative concentration of solutes inside and outside a cell, solutions can be categorized into three types:
1. Isotonic Solution:
An isotonic solution has the same solute concentration as the inside of a cell. There's no net movement of water across the cell membrane, and the cell maintains its size and shape. This is the ideal environment for many cells.
2. Hypotonic Solution:
A hypotonic solution has a lower solute concentration than the inside of a cell. This means it has a higher water concentration. Water will move into the cell by osmosis, causing the cell to swell and potentially burst (lysis) in animal cells. Plant cells, however, have a rigid cell wall that prevents bursting. Instead, they become turgid, which is important for maintaining their structural integrity.
3. Hypertonic Solution:
A hypertonic solution has a higher solute concentration than the inside of a cell. Water will move out of the cell by osmosis, causing the cell to shrink (crenation) in animal cells. Plant cells undergo plasmolysis, where the cell membrane pulls away from the cell wall, causing wilting.
Osmosis in Biological Systems:
Osmosis plays a vital role in numerous biological processes:
1. Water Absorption in Plants:
Plants absorb water from the soil through their roots via osmosis. The soil water has a higher water potential than the cells in the roots, causing water to move into the plant. This process is essential for plant growth and survival.
2. Water Regulation in Animals:
Animals maintain water balance through various mechanisms involving osmosis. For example, the kidneys regulate the concentration of solutes in the blood, ensuring proper water reabsorption.
3. Nutrient Uptake in Cells:
Osmosis facilitates the uptake of nutrients and other essential molecules by cells. The concentration gradient created by osmosis helps drive the movement of these substances across cell membranes.
4. Cell Turgor Pressure:
In plant cells, osmosis maintains cell turgor pressure, the pressure exerted by the cell contents against the cell wall. This pressure is essential for maintaining plant structure and rigidity.
Osmosis in Everyday Life:
While often studied in a biological context, osmosis has applications beyond living organisms:
1. Food Preservation:
Osmosis is used in food preservation techniques like salting or sugaring. High concentrations of salt or sugar draw water out of microorganisms, preventing their growth and spoilage.
2. Water Purification:
Reverse osmosis is a water purification technique that uses pressure to force water across a semipermeable membrane, removing impurities.
3. Medical Applications:
Osmosis is important in various medical applications, such as dialysis, where waste products are removed from the blood by diffusion across a semipermeable membrane.
Factors Affecting the Rate of Osmosis:
Several factors can influence the rate of osmosis:
- Temperature: Higher temperatures generally increase the rate of osmosis as molecules move faster.
- Concentration gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
- Surface area: A larger surface area of the membrane increases the rate of osmosis.
- Membrane permeability: The permeability of the membrane influences the rate of osmosis; more permeable membranes allow for faster water movement.
- Distance: The distance the water needs to travel across the membrane impacts the rate; shorter distances result in faster osmosis.
Conclusion:
Osmosis, the movement of water across a semipermeable membrane, is a fundamental process with far-reaching implications. From the smallest cells to large-scale water purification systems, understanding osmosis is crucial for comprehending various biological and technological processes. The interplay between water potential, osmotic pressure, and the nature of the semipermeable membrane dictates the direction and rate of water movement, significantly impacting the function and survival of living organisms and influencing various practical applications. Further research and exploration of this process continue to reveal its complexity and significance in our world.
Latest Posts
Latest Posts
-
Which Base Is Not Found In Rna
Mar 18, 2025
-
So Long To Pinky Here Comes The Thumb
Mar 18, 2025
-
When Two Amino Acids Are Joined Together
Mar 18, 2025
-
What Is The Difference Between Chemical Reaction And Nuclear Reaction
Mar 18, 2025
-
How To Write Mass Balance Equations
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Movement Of Water Across A Semipermeable Membrane Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
