The Shape Of A Protein Molecule Is Determined Completely By
Muz Play
Mar 15, 2025 · 6 min read
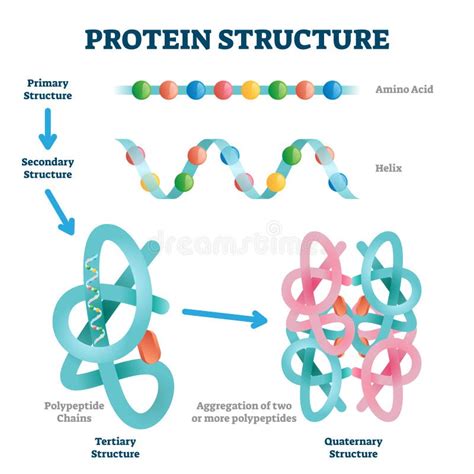
Table of Contents
The Shape of a Protein Molecule: Determined Completely by its Amino Acid Sequence
The intricate three-dimensional structure of a protein molecule is not arbitrary; it's a direct consequence of its amino acid sequence. This fundamental principle, known as the Anfinsen dogma, dictates that the information necessary for a protein to fold into its unique, biologically active conformation is encoded entirely within its linear sequence of amino acids. Understanding this relationship is crucial for comprehending protein function, disease mechanisms, and the development of novel therapeutics. This article will delve into the complexities of protein folding, exploring the various forces and factors that contribute to the precise shaping of these vital biomolecules.
The Building Blocks: Amino Acids and Peptide Bonds
Proteins are linear polymers composed of amino acids linked together by peptide bonds. Each amino acid possesses a central carbon atom (the α-carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and a unique side chain (R-group). These side chains vary significantly in size, charge, polarity, and hydrophobicity, imparting distinct chemical properties to each amino acid.
The Peptide Bond: A Crucial Link
The formation of a peptide bond occurs through a dehydration reaction between the carboxyl group of one amino acid and the amino group of another. This bond has partial double-bond character, restricting rotation around the bond and influencing the protein's overall conformation. The sequence of amino acids, dictated by the genetic code, ultimately determines the unique primary structure of the protein.
Levels of Protein Structure: From Primary to Quaternary
Protein structure is hierarchically organized into four levels:
1. Primary Structure: The Amino Acid Sequence
The primary structure is simply the linear sequence of amino acids, written from the N-terminus (amino group) to the C-terminus (carboxyl group). This sequence is directly determined by the gene encoding the protein. Any change in this sequence, even a single amino acid substitution, can dramatically alter the protein's structure and function, as seen in sickle cell anemia, where a single amino acid change in hemoglobin leads to a debilitating disease.
2. Secondary Structure: Local Folding Patterns
Secondary structure refers to local, regular folding patterns stabilized by hydrogen bonds between the backbone amide and carbonyl groups. The two most common secondary structures are:
-
α-helices: Right-handed coiled structures stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of the amino acid four residues down the chain. The side chains extend outwards from the helix.
-
β-sheets: Extended polypeptide chains arranged side-by-side, forming a pleated sheet structure. Hydrogen bonds stabilize the interaction between adjacent strands, which can be parallel or antiparallel depending on the direction of the polypeptide chains. The side chains extend alternately above and below the plane of the sheet.
Other less common secondary structures, such as loops, turns, and random coils, also contribute to the overall protein architecture.
3. Tertiary Structure: The Three-Dimensional Arrangement
Tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain, including its secondary structural elements. This structure is stabilized by a variety of weak interactions, including:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, minimizing their contact with water.
-
Hydrogen bonds: These bonds form between polar side chains and the surrounding water molecules or other polar groups within the protein.
-
Ionic interactions (salt bridges): These occur between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds formed between cysteine residues, creating strong links within the protein structure.
The tertiary structure is crucial for protein function, as it brings specific amino acid residues together to form the active site of enzymes or binding sites for other molecules.
4. Quaternary Structure: The Assembly of Subunits
Some proteins consist of multiple polypeptide chains, or subunits, assembled together to form a functional complex. This arrangement is known as the quaternary structure. The subunits are held together by the same types of interactions that stabilize tertiary structure. Examples of proteins with quaternary structure include hemoglobin, which consists of four subunits, and many enzymes that require multiple subunits for catalytic activity.
Factors Influencing Protein Folding
While the amino acid sequence dictates the final folded structure, the folding process is a complex and dynamic one, influenced by several factors:
1. Chaperones: Guiding the Folding Pathway
Chaperone proteins assist in the proper folding of other proteins, preventing aggregation and misfolding. They provide a protective environment for nascent polypeptide chains, preventing them from becoming trapped in non-native conformations. Different chaperone families utilize various mechanisms to facilitate correct folding, including preventing aggregation, promoting folding intermediates, and aiding in refolding of misfolded proteins.
2. The Cellular Environment: Impact on Folding
The cellular environment plays a crucial role in protein folding. Factors such as temperature, pH, and the presence of ions or other molecules can influence the folding process. Changes in these conditions can lead to protein denaturation, where the protein unfolds and loses its functional conformation.
3. Post-Translational Modifications: Fine-Tuning the Structure
Post-translational modifications, such as glycosylation, phosphorylation, and ubiquitination, can alter the protein's structure and function. These modifications often occur after the protein has folded into its tertiary structure and can influence its stability, activity, and interactions with other molecules.
Protein Misfolding and Disease
Errors in protein folding can have significant consequences, leading to the accumulation of misfolded proteins, which can be toxic to cells and contribute to various diseases, including:
-
Alzheimer's disease: Accumulation of amyloid plaques formed by misfolded amyloid-β protein.
-
Parkinson's disease: Aggregation of misfolded α-synuclein.
-
Cystic fibrosis: Misfolding of the cystic fibrosis transmembrane conductance regulator (CFTR) protein.
-
Prion diseases: Misfolding of prion proteins leading to the formation of infectious aggregates.
Predicting Protein Structure: Computational Approaches
Predicting protein structure from its amino acid sequence remains a significant challenge in computational biology. While significant progress has been made, accurately predicting the three-dimensional structure of complex proteins remains computationally intensive. However, advancements in computational methods, such as AlphaFold, have revolutionized the field, achieving remarkable accuracy in predicting protein structures.
Conclusion
The shape of a protein molecule is indeed determined completely by its amino acid sequence. This fundamental principle underpins our understanding of protein function, the intricacies of biological processes, and the development of diseases. The interplay of various forces, from peptide bonds to weak interactions, alongside the influence of chaperones and the cellular environment, shapes the final three-dimensional structure. Understanding this complex process remains a central focus in biological research, with implications for developing new therapeutics and tackling protein-related diseases. The future holds the promise of increasingly accurate protein structure prediction and a deeper understanding of the intricate relationship between amino acid sequence and protein conformation. Further research into protein folding mechanisms and the factors that contribute to misfolding will undoubtedly lead to significant advancements in medicine and biotechnology. The journey from a linear sequence of amino acids to a complex, functional protein remains a fascinating and ongoing area of scientific inquiry, with profound implications for our understanding of life itself.
Latest Posts
Latest Posts
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about The Shape Of A Protein Molecule Is Determined Completely By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
