The Simple Diffusion Of Water Is Also Called
Muz Play
Mar 15, 2025 · 7 min read
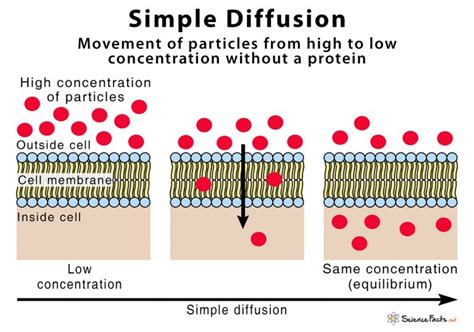
Table of Contents
The Simple Diffusion of Water is Also Called Osmosis: A Deep Dive
The simple diffusion of water across a selectively permeable membrane is also called osmosis. Understanding osmosis is crucial in various fields, from biology and medicine to environmental science and agriculture. This comprehensive guide will explore osmosis in detail, examining its mechanisms, applications, and significance across diverse contexts. We'll delve into the driving force behind osmosis, its implications for living organisms, and the factors influencing its rate.
Understanding Osmosis: A Definition
Osmosis is a passive transport process where water molecules move across a selectively permeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. Crucially, the membrane's selective permeability is key; it allows water molecules to pass but restricts the movement of most solutes. This selective permeability is often due to the presence of specific proteins or pores within the membrane.
Think of it like this: imagine you have two compartments separated by a sieve. One compartment contains a high concentration of sugar dissolved in water, while the other contains pure water. The sieve acts as the selectively permeable membrane, allowing water molecules to pass but preventing the sugar molecules from crossing. Water will naturally move from the compartment with pure water (higher water concentration) to the compartment with the sugar solution (lower water concentration) until the concentration of water is equalized on both sides. This equalization, however, doesn't mean the sugar concentration becomes equal.
The Driving Force Behind Osmosis: Water Potential
The driving force behind osmosis is water potential. Water potential is the tendency of water to move from one area to another. It's affected by several factors, primarily:
-
Solute potential (Ψs): This refers to the reduction in water potential due to the presence of dissolved solutes. The more solutes present, the lower the solute potential, and the lower the tendency for water to enter that solution. Pure water has a solute potential of zero.
-
Pressure potential (Ψp): This represents the physical pressure exerted on water. Positive pressure (pressure potential > 0) increases water potential, while negative pressure (pressure potential < 0) decreases it. Turgor pressure within plant cells is a good example of positive pressure potential.
The total water potential (Ψ) is the sum of solute potential and pressure potential: Ψ = Ψs + Ψp. Water will always move from an area of higher water potential to an area of lower water potential.
Osmotic Pressure: A Related Concept
Closely related to water potential is osmotic pressure. Osmotic pressure is the pressure required to prevent osmosis from occurring. In other words, it's the pressure needed to stop the net movement of water across a selectively permeable membrane. A higher solute concentration results in higher osmotic pressure because more pressure is needed to counteract the tendency of water to move into the solution.
Types of Osmotic Solutions and Their Effects on Cells
When we talk about osmosis in the context of cells, we often categorize solutions into three types based on their tonicity relative to the cell's internal environment:
-
Isotonic Solution: The solution's solute concentration is equal to the cell's internal concentration. There is no net movement of water; the cell remains in equilibrium.
-
Hypotonic Solution: The solution's solute concentration is lower than the cell's internal concentration. Water moves into the cell, causing it to swell and potentially lyse (burst) in animal cells. In plant cells, the cell wall prevents bursting; instead, the cell becomes turgid (firm).
-
Hypertonic Solution: The solution's solute concentration is higher than the cell's internal concentration. Water moves out of the cell, causing it to shrink and plasmolyze (the plasma membrane pulls away from the cell wall in plant cells).
Osmosis in Living Organisms: Vital Roles and Examples
Osmosis is essential for the survival and functioning of all living organisms. Here are some key examples:
-
Water absorption in plants: Plants absorb water from the soil through their roots via osmosis. The root cells have a lower water potential than the surrounding soil water, causing water to move into the roots.
-
Nutrient uptake in plants: Along with water, plants absorb essential mineral nutrients through osmosis. The concentration gradient established between the root cells and the soil facilitates this uptake.
-
Maintaining cell turgor pressure: Osmosis plays a crucial role in maintaining the turgor pressure within plant cells, which provides structural support and allows plants to stand upright.
-
Water balance in animals: Animal cells, especially those lining the digestive tract and kidneys, rely on osmosis to regulate water balance and maintain homeostasis. The kidneys, for example, use osmosis to reabsorb water back into the bloodstream.
-
Water reabsorption in the kidneys: The kidneys are master regulators of water balance in mammals. The collecting ducts in the nephrons are selectively permeable membranes that use osmosis to reabsorb water into the bloodstream, preventing excessive water loss in urine.
-
Maintaining blood pressure: Osmosis plays a role in maintaining blood pressure by controlling the amount of water in the blood.
Factors Affecting the Rate of Osmosis
Several factors influence the rate at which osmosis occurs:
-
Concentration gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
-
Temperature: Higher temperatures generally increase the rate of osmosis because water molecules move faster at higher temperatures.
-
Surface area of the membrane: A larger surface area allows more water molecules to cross the membrane simultaneously, increasing the rate of osmosis.
-
Membrane permeability: A more permeable membrane allows water to pass through more easily, leading to a faster rate of osmosis.
-
Thickness of the membrane: A thinner membrane offers less resistance to water movement, resulting in a faster rate of osmosis.
Applications of Osmosis: Beyond Biology
The principles of osmosis have significant applications beyond biology:
-
Water purification: Reverse osmosis is a widely used method for purifying water by forcing water through a semipermeable membrane under high pressure. This process removes impurities, such as salts, minerals, and bacteria.
-
Food preservation: Osmosis plays a role in food preservation techniques like pickling and curing. These methods often involve placing food in a hypertonic solution, drawing water out of the food and inhibiting microbial growth.
-
Agriculture: Understanding osmosis is essential for optimizing irrigation practices. Farmers can use this knowledge to adjust the water and nutrient levels in the soil to promote optimal plant growth.
-
Medicine: Osmosis is used in various medical applications, such as intravenous fluid administration, where the tonicity of the fluids is carefully controlled to maintain the body's water balance.
Osmosis vs. Diffusion: Key Differences
While both osmosis and diffusion are passive transport processes involving the movement of molecules from high concentration to low concentration, there are key differences:
| Feature | Osmosis | Diffusion |
|---|---|---|
| Substance | Water only | Any substance |
| Membrane | Selectively permeable membrane required | Can occur across a permeable membrane or without one |
| Driving Force | Water potential gradient | Concentration gradient |
Conclusion: The Ubiquity of Osmosis
Osmosis, the simple diffusion of water, is a fundamental process with far-reaching implications in various scientific disciplines and everyday life. From the cellular level to large-scale applications, understanding osmosis is crucial for addressing a wide range of challenges and developing innovative solutions. Its pervasive influence in biological systems and its practical applications highlight its significance as a central concept in our understanding of the natural world and its manipulation for human benefit. Further research continues to unveil new facets of this ubiquitous process, promising further advancements in diverse fields. The ongoing exploration of osmosis ensures its continued relevance and importance across scientific domains.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Simple Diffusion Of Water Is Also Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
