The Study Of Energy Transformations Is Called
Muz Play
Mar 27, 2025 · 7 min read
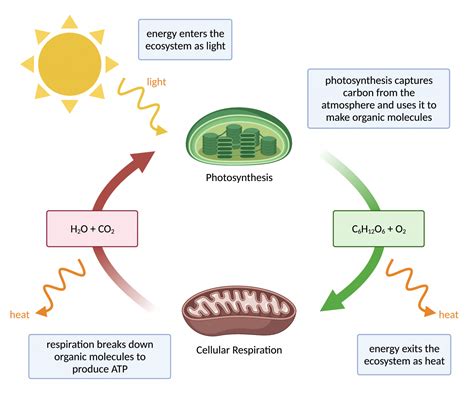
Table of Contents
The Study of Energy Transformations is Called Thermodynamics
The study of energy transformations is called thermodynamics. It's a cornerstone of physics, chemistry, and engineering, providing a framework for understanding how energy changes form and how these changes affect the world around us. From the simplest chemical reaction to the most complex biological process, thermodynamics governs the flow of energy and the resulting changes in matter. This comprehensive exploration will delve into the core principles of thermodynamics, its applications, and its profound impact on various fields.
The Fundamental Laws of Thermodynamics: Guiding Principles of Energy Transformations
Thermodynamics rests upon four fundamental laws, each providing a crucial perspective on energy and its transformations:
1. The Zeroth Law of Thermodynamics: Establishing Thermal Equilibrium
The zeroth law, though formulated later than the others, establishes the foundation for temperature measurements. It states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other. This seemingly simple statement underpins the concept of temperature as a measurable property that allows us to determine whether systems will exchange heat. Without this law, the concept of temperature wouldn't be well-defined, making the other laws less meaningful.
2. The First Law of Thermodynamics: Conservation of Energy
The first law, also known as the law of conservation of energy, asserts that energy cannot be created or destroyed, only transformed from one form to another. This means that the total energy of an isolated system remains constant. Energy can be transferred between the system and its surroundings, or converted from one form to another (e.g., chemical energy to heat energy), but the total energy remains unchanged. This law has profound implications, affecting everything from the efficiency of engines to the processes driving life itself. Understanding energy transfers is crucial in various engineering applications, such as designing efficient power plants or optimizing chemical processes.
3. The Second Law of Thermodynamics: Entropy and the Arrow of Time
The second law introduces the concept of entropy, a measure of disorder or randomness within a system. It states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. This law implies a directionality to time – processes tend to proceed towards states of higher entropy. For example, heat flows spontaneously from hot to cold objects, never the other way around, unless external work is done. This inherent irreversibility is a defining characteristic of many natural phenomena. The second law explains why some processes are spontaneous while others are not, and it imposes limitations on the efficiency of energy conversion processes, such as heat engines.
4. The Third Law of Thermodynamics: Absolute Zero
The third law states that the entropy of a perfect crystal approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15°C). This means that at absolute zero, there is no thermal energy, and the system is in its most ordered state. While absolute zero is unattainable in practice, the third law provides a reference point for measuring entropy and understanding the behavior of matter at extremely low temperatures. This law has important implications in low-temperature physics and cryogenics.
Branches and Applications of Thermodynamics
Thermodynamics is a vast field with numerous branches and applications:
Classical Thermodynamics: A Macroscopic Perspective
Classical thermodynamics deals with macroscopic properties of systems, such as temperature, pressure, volume, and entropy, without delving into the microscopic details of molecular interactions. It uses empirical relationships and mathematical models to describe the behavior of systems undergoing thermodynamic processes. This approach proves incredibly useful in analyzing engineering systems and chemical reactions.
Statistical Thermodynamics: A Microscopic Approach
Statistical thermodynamics bridges the gap between the macroscopic world of classical thermodynamics and the microscopic world of atoms and molecules. It uses statistical methods to relate macroscopic properties to the behavior of individual particles. This approach provides a deeper understanding of the underlying mechanisms driving thermodynamic processes and allows for predictions based on the microscopic characteristics of the system.
Chemical Thermodynamics: Energy in Chemical Reactions
Chemical thermodynamics focuses on the energy changes associated with chemical reactions and phase transitions. It employs concepts like enthalpy, Gibbs free energy, and equilibrium constants to predict the spontaneity and equilibrium of chemical reactions. This branch is fundamental to chemistry, materials science, and chemical engineering.
Equilibrium Thermodynamics: Steady-State Systems
Equilibrium thermodynamics deals with systems in a state of thermodynamic equilibrium, where there are no net changes in properties over time. This branch explores the relationships between thermodynamic properties and helps determine the conditions for equilibrium.
Non-Equilibrium Thermodynamics: Systems Far From Equilibrium
Non-equilibrium thermodynamics handles systems that are not in equilibrium, such as those undergoing irreversible processes. This branch studies the flow of energy and matter in systems that are far from equilibrium, and it's crucial for understanding biological systems, chemical reactors, and many other complex phenomena.
Applications Across Disciplines
The principles of thermodynamics are essential to countless fields:
Engineering: Power Generation and Efficiency
Thermodynamics forms the basis of power generation technologies, such as steam turbines, internal combustion engines, and jet engines. Engineers use thermodynamic principles to optimize engine efficiency, minimize energy losses, and design sustainable power systems. Understanding heat transfer and energy conversion is critical in designing efficient and reliable power plants.
Chemistry: Reaction Spontaneity and Equilibrium
In chemistry, thermodynamics predicts whether a chemical reaction will occur spontaneously, the equilibrium conditions of the reaction, and the amount of energy released or absorbed. This is crucial for designing and optimizing chemical processes in various industries, including pharmaceuticals, petrochemicals, and materials science.
Biology: Living Systems and Metabolism
Thermodynamics plays a crucial role in understanding biological processes. Metabolism, the complex network of chemical reactions within living organisms, is governed by thermodynamic principles. Understanding energy transfer and entropy changes is vital for comprehending the functioning of living systems.
Environmental Science: Energy Flows and Climate Change
Thermodynamics provides insights into energy flows in the environment and the impact of human activities on the climate. Studying energy balances in ecosystems helps in understanding climate change and its consequences.
Materials Science: Material Properties and Phase Transitions
Thermodynamics plays a key role in understanding the properties of materials and the transitions between different phases (solid, liquid, gas). This is vital for designing and developing new materials with specific properties for various applications.
Further Exploration of Thermodynamic Concepts
Heat Capacity and Specific Heat: Measuring Thermal Energy Storage
Heat capacity measures how much heat energy is required to raise the temperature of a substance. Specific heat is a related concept that specifies the heat capacity per unit mass. These properties are crucial in various engineering and scientific applications, enabling the calculation of energy transfers during temperature changes.
Enthalpy: Heat Content of a System
Enthalpy represents the total heat content of a system at constant pressure. It’s a key parameter in determining the heat absorbed or released during chemical reactions and phase transitions. A positive enthalpy change indicates an endothermic process (heat is absorbed), while a negative change indicates an exothermic process (heat is released).
Entropy: A Measure of Disorder
Entropy, a crucial concept in the second law of thermodynamics, quantifies the disorder or randomness within a system. An increase in entropy reflects an increase in disorder, which is a natural tendency for isolated systems. Understanding entropy changes is essential for predicting the spontaneity of processes and the efficiency of energy conversion.
Gibbs Free Energy: Spontaneity and Equilibrium
Gibbs free energy combines enthalpy and entropy to predict the spontaneity of a process at constant temperature and pressure. A negative Gibbs free energy change indicates a spontaneous process, while a positive change indicates a non-spontaneous process. Gibbs free energy is a powerful tool for analyzing chemical reactions and phase transitions.
Thermodynamic Potentials: Predicting System Behavior
Thermodynamic potentials, such as internal energy, enthalpy, Gibbs free energy, and Helmholtz free energy, provide alternative ways to describe the state of a system and predict its behavior under various conditions. These potentials are useful for analyzing different types of thermodynamic processes.
Conclusion: Thermodynamics – A Universal Framework
The study of energy transformations, thermodynamics, provides a universal framework for understanding the physical world. Its laws and concepts are fundamental to a vast array of disciplines, impacting our understanding of everything from the efficiency of engines to the intricate processes sustaining life. The continued exploration and refinement of thermodynamic principles will undoubtedly play a crucial role in addressing some of the most pressing challenges of our time, including sustainable energy development, climate change mitigation, and advancements in materials science and medicine. As we strive towards a more sustainable and technologically advanced future, understanding and applying the principles of thermodynamics remains increasingly important.
Latest Posts
Latest Posts
-
Charging And Discharging Of Capacitor Formula
Mar 30, 2025
-
When Do You Use Henderson Hasselbalch Equation
Mar 30, 2025
-
What Happens To The Temperature During A Phase Change
Mar 30, 2025
-
A Tentative Explanation For An Event Which Can Be Testabl
Mar 30, 2025
-
How To Check If A Matrix Is Invertible
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about The Study Of Energy Transformations Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
