The Substance That Dissolves The Solute
Muz Play
Mar 23, 2025 · 6 min read
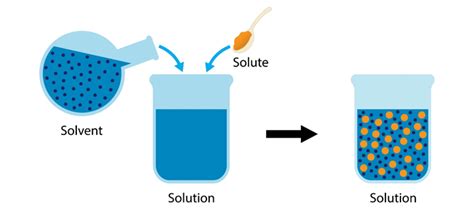
Table of Contents
The Substance That Dissolves the Solute: A Deep Dive into Solvents
The seemingly simple question, "What is the substance that dissolves the solute?" belies a rich and complex world of chemistry. The answer, of course, is the solvent. But understanding solvents goes far beyond a simple definition. This exploration delves into the properties of solvents, their classification, applications, and the fascinating science behind the solvation process.
Understanding Solvents: More Than Just a Dissolver
A solvent is a substance that dissolves a solute, resulting in a solution. The solute is the substance being dissolved, and the resulting homogeneous mixture is the solution. Think of making sweet tea: the water is the solvent, the sugar is the solute, and the resulting sweet tea is the solution. This seems straightforward, but the nature of the solvent-solute interaction dictates the solubility and the properties of the final solution.
Key Properties of Solvents
Several key properties determine a solvent's effectiveness and suitability for a particular application. These include:
-
Polarity: This is arguably the most crucial property. Polar solvents possess a dipole moment – a separation of positive and negative charges within the molecule. Water (H₂O) is a prime example of a polar solvent, with its oxygen atom carrying a partial negative charge and its hydrogen atoms carrying partial positive charges. Polar solvents effectively dissolve polar solutes (like sugars and salts) due to strong dipole-dipole interactions.
-
Proticity: Protic solvents possess a hydrogen atom bonded to an electronegative atom (like oxygen or nitrogen). These hydrogen atoms can form hydrogen bonds with solute molecules, further enhancing solubility. Water, alcohols, and carboxylic acids are examples of protic solvents. Aprotic solvents lack this hydrogen bonding capability. Examples include acetone, diethyl ether, and acetonitrile.
-
Viscosity: This refers to a solvent's resistance to flow. High viscosity solvents can hinder the dissolution process, while low viscosity solvents facilitate faster dissolution.
-
Boiling Point: The boiling point influences the ease of solvent removal after the desired reaction or process is complete. Lower boiling point solvents are preferred for easy evaporation.
-
Toxicity and Safety: The toxicity and safety of a solvent are crucial considerations, particularly in industrial and laboratory settings. Many solvents are volatile and flammable, requiring careful handling and appropriate safety measures.
Classifying Solvents: A Diverse World
Solvents are incredibly diverse and can be classified in several ways:
Based on Polarity:
-
Polar Solvents: These dissolve polar solutes and are often miscible with water (meaning they mix freely). Examples include water, alcohols (methanol, ethanol), ketones (acetone), and acetonitrile.
-
Nonpolar Solvents: These dissolve nonpolar solutes and are generally immiscible with water (meaning they don't mix). Examples include hexane, benzene, toluene, and diethyl ether.
Based on Proticity:
-
Protic Solvents: As mentioned earlier, these solvents possess a hydrogen atom bonded to an electronegative atom capable of hydrogen bonding. They are excellent for dissolving ionic compounds and polar molecules.
-
Aprotic Solvents: These solvents lack the hydrogen bonding capability of protic solvents. They are often used in reactions where hydrogen bonding might interfere with the process.
Based on Chemical Structure:
Solvents can also be classified based on their chemical structure, such as alcohols, ketones, ethers, esters, hydrocarbons, and more. Each class exhibits distinct properties influencing their solvation capabilities.
The Solvation Process: A Molecular Dance
The process of dissolution, also known as solvation, is a complex interplay of intermolecular forces. When a solute dissolves in a solvent, the solvent molecules surround the solute molecules, effectively breaking apart the solute's structure. This process is driven by the thermodynamic principle of minimizing the Gibbs free energy.
Intermolecular Forces at Play:
Several intermolecular forces are involved in the solvation process:
-
Ion-dipole interactions: These occur between ions (charged particles) and polar molecules. This is a dominant force in the dissolution of ionic compounds in polar solvents like water.
-
Dipole-dipole interactions: These occur between two polar molecules. The positive end of one molecule is attracted to the negative end of the other.
-
Hydrogen bonding: A special type of dipole-dipole interaction involving hydrogen atoms bonded to electronegative atoms. It's a strong intermolecular force that plays a significant role in the solubility of many molecules in water and other protic solvents.
-
London dispersion forces: These are weak intermolecular forces that exist between all molecules, regardless of polarity. They are the primary intermolecular force in nonpolar solvents and solutes.
The Energetics of Solvation:
Solvation is an energetic process involving both endothermic (heat-absorbing) and exothermic (heat-releasing) steps. The overall process is favored if the decrease in enthalpy (heat content) and the increase in entropy (disorder) lead to a net decrease in the Gibbs free energy. If the Gibbs free energy increase, the solute will not dissolve readily.
Applications of Solvents: A Wide Spectrum
Solvents are ubiquitous and find applications in a vast array of fields:
Industrial Applications:
-
Cleaning: Solvents are crucial for cleaning and degreasing various surfaces and components in manufacturing and maintenance.
-
Coatings: Solvents are used as carriers for paints, varnishes, and other coatings.
-
Extraction: Solvents are employed in the extraction of valuable substances from natural resources.
-
Chemical Synthesis: Solvents provide the reaction medium for countless chemical reactions.
Laboratory Applications:
-
Chromatography: Solvents are essential in chromatography techniques used to separate and analyze mixtures.
-
Spectroscopy: Solvents are used as the medium for various spectroscopic analyses.
-
Sample Preparation: Solvents play a critical role in sample preparation for various analytical techniques.
Everyday Applications:
-
Paints and thinners: These contain solvents that help with application and drying.
-
Cleaning products: Many cleaning products utilize solvents to dissolve grease and grime.
-
Personal care products: Solvents are often included in cosmetics, perfumes, and other personal care products.
Choosing the Right Solvent: A Matter of Compatibility
Selecting the appropriate solvent for a specific application is crucial and depends on several factors:
-
The nature of the solute: Polar solutes require polar solvents, while nonpolar solutes require nonpolar solvents. "Like dissolves like" is a fundamental principle in solubility.
-
The desired application: The solvent's boiling point, viscosity, toxicity, and flammability must be considered based on the application.
-
Environmental concerns: The environmental impact of the solvent must be taken into account, favoring less harmful and more biodegradable alternatives.
Green Solvents: A Sustainable Future
Increasing environmental awareness has led to a growing interest in green solvents – solvents that are less harmful to the environment than traditional organic solvents. These solvents are often derived from renewable resources, are biodegradable, and have low toxicity. Examples include supercritical carbon dioxide, ionic liquids, and water.
Conclusion: The Solvent's Unsung Role
While often overlooked, the solvent plays a crucial and multifaceted role in numerous chemical and industrial processes. Understanding its properties, classification, and the underlying principles of solvation is essential for anyone working in chemistry, chemical engineering, or related fields. The ongoing quest for environmentally friendly and efficient solvents highlights the enduring importance of this fundamental chemical substance. Future research will undoubtedly continue to refine our understanding of solvation and expand the range of applications for these essential materials.
Latest Posts
Latest Posts
-
Metals Are Located Where On The Periodic Table
Mar 25, 2025
-
All Organisms Are Composed Of One Or More Cells
Mar 25, 2025
-
Anything That Interferes With Successful Communication Is Said To Be
Mar 25, 2025
-
Construct The Confidence Interval For The Population Mean M
Mar 25, 2025
-
Is Mixing A Chemical Or Physical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Substance That Dissolves The Solute . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
