The Units Of Molar Mass Are
Muz Play
Mar 18, 2025 · 6 min read
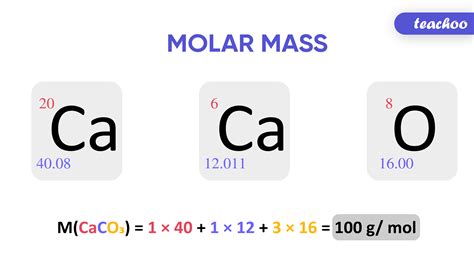
Table of Contents
The Units of Molar Mass: A Deep Dive into the Foundation of Chemistry
Molar mass, a cornerstone concept in chemistry, represents the mass of one mole of a substance. Understanding its units is crucial for accurate calculations and a solid grasp of stoichiometry. This article delves deep into the units of molar mass, exploring their significance, derivations, and applications across various chemical calculations. We’ll also examine the relationship between molar mass and other essential chemical concepts like Avogadro's number and atomic mass.
Defining Molar Mass: More Than Just a Number
Before diving into the units, let's solidify our understanding of molar mass itself. Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). A mole, denoted by the symbol "mol," is a fundamental unit in the International System of Units (SI) and represents a specific number of particles: Avogadro's number, approximately 6.022 x 10<sup>23</sup>. Therefore, the molar mass tells us the mass of 6.022 x 10<sup>23</sup> atoms, molecules, ions, or formula units of a substance.
For example, the molar mass of carbon (C) is approximately 12.01 g/mol. This means that one mole of carbon atoms weighs 12.01 grams, and that 6.022 x 10<sup>23</sup> carbon atoms collectively have a mass of 12.01 grams.
The Units: Grams per Mole (g/mol) – A Detailed Explanation
The standard unit for molar mass is grams per mole (g/mol). Let's break down why this specific unit is used:
-
Grams (g): This is the unit of mass in the SI system. It represents the amount of matter in a substance. Using grams ensures consistency and compatibility with other chemical measurements.
-
Mole (mol): As discussed earlier, the mole is a unit representing a specific number of particles. Using moles allows for easy conversion between the macroscopic world (grams) and the microscopic world (number of atoms or molecules). This is vital in stoichiometric calculations.
The combination of grams and moles in the unit g/mol provides a clear and concise way to express the mass of a substance relative to the number of particles it contains. This ratio is essential for numerous calculations, including:
-
Converting grams to moles and vice versa: This fundamental conversion is essential for solving various stoichiometry problems.
-
Determining the empirical and molecular formulas: Knowing the molar mass allows chemists to determine the empirical and molecular formulas of unknown compounds.
-
Calculating reaction yields: Molar mass is crucial in calculating the theoretical yield and percent yield of chemical reactions.
-
Determining the concentration of solutions: Molarity, a common unit of concentration, is defined as moles of solute per liter of solution, directly relying on the concept of molar mass.
Beyond g/mol: Other Representations and Contextual Units
While g/mol is the primary unit, other representations can be used depending on the context:
-
Kilograms per mole (kg/mol): This unit is sometimes used, particularly when dealing with larger quantities of substances or in specific applications. It’s simply a conversion of g/mol, with 1 kg/mol being equal to 1000 g/mol.
-
Atomic Mass Units (amu): While not strictly a unit of molar mass, amu is closely related. The atomic mass of an element, often expressed in amu, is numerically equivalent to its molar mass in g/mol. One amu is approximately the mass of one proton or neutron.
-
Relative Molecular Mass (Mr): This term is often used for compounds, and it represents the sum of the relative atomic masses of all atoms in a molecule. It is a dimensionless quantity, but it directly relates to the molar mass. The numerical value of Mr is the same as the molar mass in g/mol.
Calculating Molar Mass: A Step-by-Step Guide
Calculating the molar mass involves summing the atomic masses of all atoms present in a chemical formula. Let's illustrate this with a few examples:
Example 1: Water (H₂O)
- Atomic mass of Hydrogen (H): ~1.01 g/mol
- Atomic mass of Oxygen (O): ~16.00 g/mol
Molar mass of H₂O = (2 * 1.01 g/mol) + (1 * 16.00 g/mol) = 18.02 g/mol
Example 2: Sodium Chloride (NaCl)
- Atomic mass of Sodium (Na): ~22.99 g/mol
- Atomic mass of Chlorine (Cl): ~35.45 g/mol
Molar mass of NaCl = (1 * 22.99 g/mol) + (1 * 35.45 g/mol) = 58.44 g/mol
Example 3: Glucose (C₆H₁₂O₆)
- Atomic mass of Carbon (C): ~12.01 g/mol
- Atomic mass of Hydrogen (H): ~1.01 g/mol
- Atomic mass of Oxygen (O): ~16.00 g/mol
Molar mass of C₆H₁₂O₆ = (6 * 12.01 g/mol) + (12 * 1.01 g/mol) + (6 * 16.00 g/mol) = 180.18 g/mol
The Importance of Precise Units and Significant Figures
Accuracy in chemical calculations is paramount. Using the correct units for molar mass, specifically g/mol, ensures consistency and prevents errors. Furthermore, paying close attention to significant figures is crucial. The number of significant figures in the calculated molar mass should reflect the precision of the atomic masses used.
Molar Mass and Avogadro's Number: The Intertwined Relationship
The concept of molar mass is intrinsically linked to Avogadro's number. Avogadro's number (approximately 6.022 x 10<sup>23</sup>) represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. The molar mass, therefore, provides a bridge between the mass of a substance and the number of particles it contains. This relationship is crucial for performing various stoichiometric calculations.
For example, if we know the molar mass of a substance and its mass in grams, we can use Avogadro's number to calculate the number of particles present.
Applications of Molar Mass: A Wide Range of Chemical Calculations
Molar mass is a fundamental concept with wide-ranging applications across various chemical calculations. Some key applications include:
-
Stoichiometry: Molar mass is used to convert between mass and moles in stoichiometric calculations, allowing us to determine the amounts of reactants and products in a chemical reaction.
-
Empirical and Molecular Formula Determination: By knowing the molar mass and the elemental composition (percent composition) of a compound, one can determine its empirical and molecular formulas.
-
Solution Concentration Calculations: Molarity, molality, and other concentration units rely heavily on the concept of molar mass.
-
Thermochemistry: In thermochemical calculations, molar mass is often used to convert between the energy change per mole and the energy change per gram.
-
Gas Laws: The ideal gas law and other gas laws often involve using moles, which are directly calculated using molar mass.
Conclusion: Mastering Molar Mass for Chemical Proficiency
Understanding the units of molar mass – primarily grams per mole (g/mol) – is essential for success in chemistry. This unit provides a crucial link between the macroscopic world of measurable mass and the microscopic world of atoms and molecules. Its significance extends far beyond simple definitions, playing a pivotal role in a wide array of chemical calculations, from basic stoichiometry to complex thermodynamic and kinetic analyses. By grasping the meaning and applications of molar mass and its units, you lay a strong foundation for further exploration in the fascinating world of chemistry. Precise calculations and a thorough understanding of the underlying principles will unlock deeper insights into chemical reactions and behavior.
Latest Posts
Latest Posts
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
-
What Two Regions Make Up All Atoms
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Units Of Molar Mass Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
