The Vertical Column Of The Periodic Table
Muz Play
Mar 20, 2025 · 6 min read
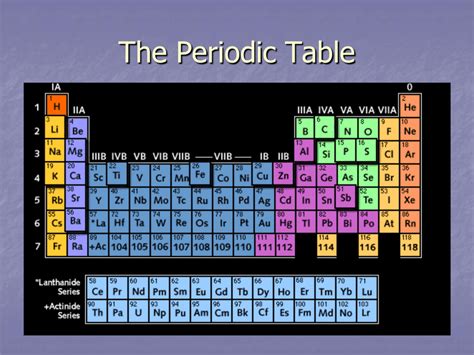
Table of Contents
Delving Deep into the Vertical Columns of the Periodic Table: Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. While horizontal rows (periods) showcase the gradual filling of electron shells, the vertical columns, known as groups or families, reveal a more profound similarity – elements within the same group share strikingly similar chemical behavior. This article dives deep into the fascinating world of these vertical columns, exploring their unique characteristics, trends, and the underlying reasons for their shared properties.
Understanding Group Properties: A Shared Electron Configuration Story
The defining characteristic of elements within a group is their similar valence electron configuration. Valence electrons are the outermost electrons in an atom, playing a crucial role in chemical bonding. Elements in the same group possess the same number of valence electrons, leading to predictable similarities in their reactivity and bonding patterns. This shared electron configuration dictates how they interact with other elements, forming compounds with similar structures and properties.
For example, Group 1, the alkali metals (Li, Na, K, Rb, Cs, Fr), all have one valence electron. This single electron is easily lost, resulting in a +1 charge and highly reactive nature. This shared characteristic explains why all alkali metals readily react with water, producing hydrogen gas and a hydroxide. Similarly, Group 18, the noble gases (He, Ne, Ar, Kr, Xe, Rn), possess a full valence shell, making them exceptionally unreactive and stable. This full valence shell explains their inert nature and lack of readily forming compounds.
The Significance of Valence Electrons in Chemical Bonding
The number of valence electrons directly influences an element's electronegativity, ionization energy, and atomic radius. These properties are fundamental in determining the type of chemical bonds an element forms (ionic, covalent, metallic) and the strength of those bonds.
-
Electronegativity: This measures an atom's ability to attract electrons in a chemical bond. Generally, electronegativity decreases down a group as atomic size increases, and the outermost electrons are further from the nucleus and less strongly attracted.
-
Ionization Energy: This represents the energy required to remove an electron from an atom. Ionization energy typically decreases down a group because the increasing atomic size results in weaker attraction between the nucleus and the valence electrons.
-
Atomic Radius: The size of an atom generally increases down a group due to the addition of electron shells. This increased distance between the nucleus and valence electrons weakens the electrostatic attraction, impacting chemical behavior.
Exploring the Major Groups: A Detailed Look
The periodic table is often divided into different blocks based on the subshell being filled (s, p, d, f). Let's explore some of the key groups in detail:
Group 1: The Alkali Metals
As mentioned previously, alkali metals are highly reactive due to their single valence electron. Their reactivity increases down the group as the ionization energy decreases. They readily form +1 ions and react vigorously with water, oxygen, and halogens.
Key characteristics:
- Soft, silvery-white metals: Easily cut with a knife.
- Low melting and boiling points: Compared to other metals.
- Excellent conductors of heat and electricity: Due to their mobile valence electrons.
- Form ionic compounds: Readily losing their valence electron to achieve a stable octet.
Group 2: The Alkaline Earth Metals
Alkaline earth metals have two valence electrons, making them less reactive than alkali metals but still significantly reactive compared to other groups. They form +2 ions and are essential components in many biological processes and industrial applications.
Key characteristics:
- Slightly harder and denser than alkali metals: Possessing higher melting and boiling points.
- Reactive, but less so than alkali metals: Reacting with water (though often less vigorously than alkali metals).
- Form ionic compounds: Losing their two valence electrons.
- Important biological roles: Calcium and magnesium are particularly crucial in living organisms.
Group 17: The Halogens
Halogens are highly reactive nonmetals with seven valence electrons. They readily gain one electron to form a -1 ion, achieving a stable octet. Their reactivity decreases down the group as the atomic size increases, and the added electron is less strongly attracted to the nucleus.
Key characteristics:
- Highly reactive nonmetals: Reacting readily with metals to form salts.
- Diatomic molecules: Exist as pairs in their elemental form (e.g., Cl₂, Br₂).
- Varying physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperature.
- Essential in biological processes and industrial applications: Chlorine is used in water purification, and iodine is essential for thyroid function.
Group 18: The Noble Gases
The noble gases are inert and unreactive due to their complete valence shells. They are monatomic gases and have very low boiling points. Historically considered inert, recent research has shown that some noble gases can form compounds under specific conditions, challenging the "inert" label.
Key characteristics:
- Extremely unreactive: Due to their full valence shells.
- Monatomic gases: Exist as single atoms.
- Low boiling points: Reflecting weak interatomic forces.
- Used in various applications: Helium in balloons, neon in lighting, argon in welding.
Transition Metals: A Special Case
The transition metals occupy the d-block of the periodic table, exhibiting a complex range of properties due to their partially filled d orbitals. They are characterized by variable oxidation states, often forming colored compounds, and exhibiting catalytic activity. Their properties are less predictable than those in the main group elements, and the trends are not as straightforward.
Beyond the Main Groups: The Lanthanides and Actinides
The lanthanides (rare earth elements) and actinides occupy the f-block of the periodic table. They exhibit similar chemical properties within their respective series, primarily due to the filling of their 4f and 5f orbitals. Many are radioactive, and their properties are less well-understood compared to the main group elements and transition metals.
Applications and Importance
The unique properties of elements within each group lead to a wide range of applications across various industries and fields. Understanding these properties is crucial in designing materials with specific characteristics, developing new technologies, and addressing societal challenges. For instance:
- Alkali metals are used in batteries and lighting.
- Alkaline earth metals are essential in construction materials (cement, etc.) and biological processes.
- Halogens find applications in disinfectants, pharmaceuticals, and industrial processes.
- Noble gases have various applications in lighting, welding, and cryogenics.
- Transition metals are vital in catalysis, metallurgy, and electronics.
Conclusion: The Power of Organization and Prediction
The vertical columns of the periodic table are more than just a convenient organizational tool. They represent a deep understanding of the underlying principles governing atomic structure and chemical behavior. The similarities in valence electron configuration within a group allow for the prediction of chemical properties, reactivity, and bonding patterns. This knowledge is invaluable in various scientific and technological fields, driving innovation and shaping our understanding of the material world. Continued research and exploration into the properties of these groups will undoubtedly lead to further advancements and discoveries in the future. The periodic table, with its carefully organized columns, stands as a testament to the elegance and power of scientific organization, allowing us to unravel the mysteries of the elements and harness their unique properties for the betterment of humankind.
Latest Posts
Latest Posts
-
Calculating The Ph At The Equivalence Point
Mar 21, 2025
-
How Does Temperature Affect Diffusion Rate
Mar 21, 2025
-
What Are The Units Of Wavelength
Mar 21, 2025
-
Map North Africa And Southwest Asia
Mar 21, 2025
-
All Cells Have What Three Following Things
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about The Vertical Column Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
