Their Numbers Will Vary In Isotopes Of The Same Element.
Muz Play
Mar 15, 2025 · 6 min read
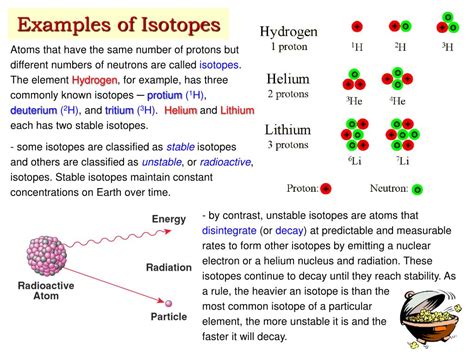
Table of Contents
Their Numbers Will Vary: Delving into Isotopes and Their Significance
The world around us is built upon the fundamental building blocks of matter: atoms. While we often visualize atoms as simple, indivisible units, the reality is far richer and more nuanced. Atoms of the same element, characterized by the same number of protons, can actually exist in different forms, differing in the number of neutrons within their nuclei. These variations are known as isotopes, and understanding their properties and behaviors is crucial in numerous scientific fields. This article will explore the fascinating world of isotopes, explaining what they are, how they differ, their applications, and their significant role in various scientific disciplines.
Understanding Isotopes: A Deeper Dive into Atomic Structure
Atoms are composed of three subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge and reside in the atom's nucleus. Neutrons, as their name suggests, are neutral and also found within the nucleus. Electrons, carrying a negative charge, orbit the nucleus in electron shells. The atomic number of an element is defined by the number of protons it possesses – this number uniquely identifies each element on the periodic table.
However, the number of neutrons in an atom's nucleus can vary. This is where isotopes come into play. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. Since the number of protons dictates the element's identity, isotopes of the same element will exhibit similar chemical properties. However, the differing neutron number can significantly impact their physical properties, such as mass and radioactivity.
Mass Number and Isotopic Notation
The mass number of an atom is the total number of protons and neutrons in its nucleus. Because isotopes differ in their neutron count, they also have different mass numbers. Isotopes are often represented using a specific notation:
^A_Z X: Where 'X' represents the element's symbol, 'Z' is the atomic number (number of protons), and 'A' is the mass number (protons + neutrons).
For example, Carbon-12 (¹²₆C) has 6 protons and 6 neutrons, while Carbon-14 (¹⁴₆C) has 6 protons and 8 neutrons. Both are isotopes of carbon, but their mass numbers and properties differ.
Isotope Abundance and Average Atomic Mass
Most elements exist in nature as a mixture of different isotopes. The isotopic abundance refers to the relative proportion of each isotope present in a naturally occurring sample of an element. These abundances are usually expressed as percentages. For example, carbon exists primarily as Carbon-12 (approximately 98.9%) and Carbon-13 (approximately 1.1%), with trace amounts of Carbon-14.
The average atomic mass of an element listed on the periodic table is a weighted average of the masses of all its naturally occurring isotopes, taking into account their relative abundances. This value reflects the average mass of an atom of that element found in nature.
The Significance of Isotopes in Various Fields
Isotopes play a crucial role in various scientific and technological disciplines. Their unique properties make them indispensable tools for research and applications across multiple fields.
1. Radiometric Dating and Archaeology
Radioactive isotopes, which undergo radioactive decay, are essential for radiometric dating. This technique is used to determine the age of geological formations, artifacts, and other materials. By measuring the ratio of a radioactive isotope to its decay product, scientists can estimate the time elapsed since the material's formation. Famous examples include Carbon-14 dating used to date organic materials and Uranium-Lead dating used for dating rocks.
2. Medical Applications
Radioactive isotopes are widely used in nuclear medicine. They are incorporated into radiotracers, which are then injected into the body to track various physiological processes. This technique is used for diagnostic imaging (like PET scans) and targeted cancer therapy. Specific isotopes like Technetium-99m and Iodine-131 are frequently employed in these applications.
3. Industrial Applications
Isotopes find applications in various industrial processes. They are used in non-destructive testing to detect flaws in materials, in gauging processes to measure the thickness of materials, and in tracer studies to monitor the flow of materials within industrial systems. For example, radioactive isotopes are used to trace the movement of water in pipes or to track the flow of oil in pipelines.
4. Environmental Science
Isotopes are essential tools in environmental science. Stable isotopes (non-radioactive isotopes) are used to study environmental processes such as water cycling, nutrient flows in ecosystems, and climate change. By analyzing the isotopic ratios in various environmental samples, scientists can gain valuable insights into past and present environmental conditions. Oxygen and hydrogen isotopes in water are commonly used for this purpose.
5. Forensic Science
Isotopic analysis has found its place in forensic science. Analyzing the isotopic composition of materials found at crime scenes can help link suspects to the crime or trace the origin of materials. For example, isotopic analysis of hair or other biological samples can help determine a person's geographic origin or dietary habits.
Isotope Effects: The Impact of Neutron Number
The differing number of neutrons in isotopes leads to variations in their physical and chemical properties, known as isotope effects. These effects, while often subtle, can have significant implications in various processes.
1. Mass Effects
The most significant isotope effect stems from the difference in mass. Heavier isotopes tend to react more slowly than lighter ones due to their increased inertia. This mass effect is particularly prominent in reactions involving diffusion or kinetic isotope effects.
2. Kinetic Isotope Effects
Kinetic isotope effects refer to the differences in reaction rates observed between isotopes of the same element. These effects arise from the mass difference affecting the vibrational frequencies of the molecules involved in the reaction. Heavier isotopes generally result in slower reaction rates.
3. Equilibrium Isotope Effects
Equilibrium isotope effects are observed in equilibrium reactions where the isotopic composition of reactants and products differs. These effects arise from the different vibrational energies of isotopic molecules influencing the equilibrium constant.
4. Spectroscopic Isotope Effects
Isotopic substitution can affect the vibrational frequencies of molecules, leading to shifts in their infrared and Raman spectra. These spectroscopic isotope effects are used to identify and characterize different isotopes within a molecule.
Isotope Separation: Techniques for Isolating Isotopes
Since many elements exist as mixtures of isotopes, various techniques are employed to separate and isolate specific isotopes. These techniques are crucial for obtaining enriched isotopes used in various applications.
Some common methods include:
-
Gaseous diffusion: This method exploits the difference in diffusion rates of gaseous isotopes. Lighter isotopes diffuse faster through a porous membrane, leading to their partial separation.
-
Centrifugation: This method utilizes centrifugal force to separate isotopes based on their mass differences. Heavier isotopes tend to concentrate towards the outer edge of the centrifuge.
-
Electromagnetic separation: This method uses electromagnetic fields to separate ions of different isotopes based on their mass-to-charge ratios.
-
Laser isotope separation: This method utilizes lasers tuned to specific wavelengths to selectively excite and ionize specific isotopes, enabling their separation.
Conclusion: The Enduring Importance of Isotopes
Isotopes, despite their subtle differences, represent a fundamental aspect of atomic structure and have profound implications across various scientific and technological fields. Their diverse applications in dating, medicine, industry, and environmental science demonstrate their indispensable role in our understanding of the world and in advancing technological progress. As research continues, the significance of isotopes will undoubtedly grow, leading to further advancements and applications in fields we haven't even imagined yet. The journey of unraveling the mysteries of isotopes is ongoing, with future discoveries promising to reveal even more about the fundamental building blocks of matter and their profound influence on our universe.
Latest Posts
Latest Posts
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Their Numbers Will Vary In Isotopes Of The Same Element. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
