Trend Of Basicity In Periodic Table
Muz Play
Mar 27, 2025 · 6 min read
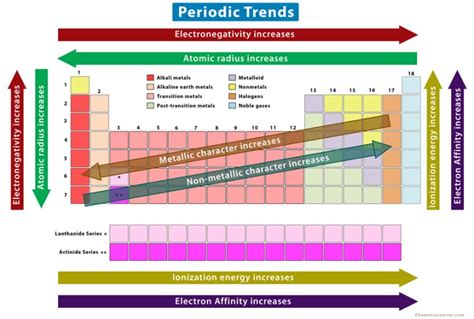
Table of Contents
The Trend of Basicity in the Periodic Table: A Comprehensive Exploration
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding these properties, and how they trend across the table, is crucial for predicting chemical behavior. One such fundamental property is basicity, the ability of a substance to accept a proton (H⁺). This article will delve deep into the trends of basicity in the periodic table, exploring the factors that influence it and examining specific examples across different groups and periods.
Factors Influencing Basicity
Several key factors determine an element's or compound's basicity. These include:
1. Electronegativity:
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Highly electronegative elements tend to form weaker bases. This is because the electronegative atom strongly attracts the bonding electrons, making it less likely to share them with a proton (H⁺). Conversely, elements with low electronegativity readily share electrons and are therefore stronger bases.
2. Atomic Size:
Larger atoms generally form stronger bases. This is because the increased distance between the nucleus and the valence electrons reduces the attraction between them. Consequently, the electrons are more readily available to share with a proton, increasing basicity.
3. Charge Density:
Charge density refers to the charge of an ion relative to its size. Higher charge density leads to weaker basicity. A highly charged ion strongly attracts electrons, making it less likely to accept a proton. Conversely, ions with lower charge density are better at accepting protons, enhancing their basicity.
4. Hybridization:
The hybridization of orbitals influences basicity, especially in organic chemistry. Different hybrid orbitals have varying degrees of s-character. Orbitals with greater s-character are more electronegative and therefore lead to weaker bases. Conversely, orbitals with less s-character are less electronegative and form stronger bases.
5. Solvation Effects:
The solvent plays a crucial role in determining the basicity of a substance. The interaction between the base and the solvent affects the availability of the lone pairs of electrons for proton acceptance. Different solvents can significantly alter the relative basicity of different compounds. Protic solvents, those containing acidic protons, can often influence basicity more significantly than aprotic solvents.
Basicity Trends Across the Periodic Table
Let's now examine basicity trends across groups and periods:
Group 1 (Alkali Metals):
Alkali metals readily lose their single valence electron, forming positively charged ions (cations). These cations are not basic; they are acidic due to their ability to polarize water molecules, increasing the concentration of H⁺ ions. The basicity is associated with their hydroxides (MOH), where the hydroxide ion (OH⁻) acts as the base. Down the group, the basicity of the hydroxides increases because of the increase in atomic size and decreased charge density. Therefore, CsOH is a stronger base than LiOH.
Group 2 (Alkaline Earth Metals):
Similar to alkali metals, alkaline earth metals primarily form cations that are not basic. However, their hydroxides (M(OH)₂) display basicity. Again, basicity increases down the group due to the increasing atomic size and decreasing charge density. Thus, Ba(OH)₂ is a stronger base than Be(OH)₂.
Group 15 (Pnictogens):
This group provides a fascinating example of changing basicity. Ammonia (NH₃) is a weak base, accepting a proton to form the ammonium ion (NH₄⁺). As we move down the group to phosphine (PH₃), arsine (AsH₃), and stibine (SbH₃), the basicity decreases significantly. This is primarily because of the decrease in electronegativity and the increase in atomic size. The larger atoms lead to weaker bonding with the proton, and the decreased electronegativity makes them less inclined to share electrons.
Group 16 (Chalcogens):
The chalcogens show a similar trend to the pnictogens. Water (H₂O) is a weak base, but its basicity is still considerably stronger than that of hydrogen sulfide (H₂S) or hydrogen selenide (H₂Se). Again, the decrease in basicity down the group is attributed to the increased size and decreased electronegativity of the chalcogen atoms.
Group 17 (Halogens):
Halogens typically act as oxidizing agents rather than bases. However, halide ions (F⁻, Cl⁻, Br⁻, I⁻) can act as weak bases. Basicity increases down the group as the size of the halide ion increases and the charge density decreases. Therefore, I⁻ is a stronger base than F⁻.
Across the Periods:
Moving across a period from left to right, basicity generally decreases. This is primarily due to the increase in electronegativity and the decrease in atomic size. The elements at the left-hand side of the period (alkali and alkaline earth metals) form the stronger bases. As you move across, the basicity decreases until you reach the halogens and noble gases, which are generally non-basic.
Examples of Basicity Trends
To illustrate the concepts discussed above, let’s look at some specific examples:
- LiOH vs. CsOH: CsOH is a stronger base than LiOH because of its larger atomic size and lower charge density.
- Mg(OH)₂ vs. Ba(OH)₂: Ba(OH)₂ is a stronger base due to the larger size and lower charge density of the barium ion.
- NH₃ vs. PH₃: NH₃ is a stronger base than PH₃ because nitrogen is more electronegative and smaller than phosphorus. The lone pair on nitrogen is more available for protonation.
- H₂O vs. H₂S: H₂O is a stronger base than H₂S because oxygen is more electronegative and smaller than sulfur.
- F⁻ vs. I⁻: I⁻ is a stronger base than F⁻ due to its larger size and lower charge density.
Practical Applications of Understanding Basicity Trends
Understanding the trends in basicity is essential in various fields:
- Inorganic Chemistry: Predicting the reactivity of compounds and designing new materials with desired properties.
- Organic Chemistry: Understanding reaction mechanisms and predicting the outcome of acid-base reactions.
- Analytical Chemistry: Developing titrations and other analytical techniques.
- Environmental Chemistry: Understanding the behavior of pollutants in the environment.
- Biochemistry: Understanding the role of bases in biological systems.
Conclusion:
The basicity of an element or compound is a complex property influenced by several interrelated factors. Understanding these factors and their trends across the periodic table is crucial for predicting and explaining chemical behavior. While general trends exist, exceptions can occur due to specific molecular structures and environmental conditions. By mastering these concepts, chemists can better design experiments, interpret results, and develop new applications in various fields. The continuing research and refinement of our understanding of basicity and its related properties remain a vital aspect of chemical science. Further study of the nuances of these trends allows for deeper insights and breakthroughs in numerous areas of chemistry and beyond.
Latest Posts
Latest Posts
-
Adding Integers With The Same Sign
Mar 30, 2025
-
How Do You Divide Fractions With Exponents
Mar 30, 2025
-
N Type Semiconductor Vs P Type Semiconductor
Mar 30, 2025
-
Bipolar Junction Transistor As A Switch
Mar 30, 2025
-
Starch Glycogen And Cellulose Are Examples Of
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Trend Of Basicity In Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
