Trend Of Melting Point In Periodic Table
Muz Play
Mar 22, 2025 · 6 min read
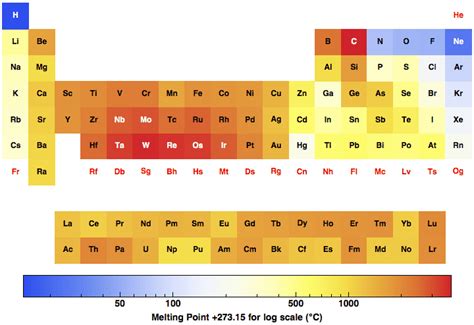
Table of Contents
The Trend of Melting Point in the Periodic Table: A Comprehensive Exploration
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. One such property, crucial for understanding material behavior, is the melting point – the temperature at which a solid transitions to a liquid. While seemingly simple, the trend of melting points across the periodic table reveals a fascinating interplay of atomic forces and structural characteristics. This exploration will delve into the factors influencing melting points, examining the trends across periods and groups, highlighting exceptions, and exploring the applications of this knowledge.
Factors Influencing Melting Point
Several key factors dictate an element's melting point. Understanding these is fundamental to interpreting the observed trends:
1. Atomic Size and Mass:
Larger atoms generally possess lower melting points. This is because the attraction between the nucleus and valence electrons weakens with increasing distance. The weaker attraction translates to weaker interatomic forces, requiring less energy to overcome them during melting. Similarly, increased atomic mass, while not directly impacting attractive forces, contributes to increased electron cloud size, indirectly reducing attractive forces and lowering the melting point.
2. Atomic Number and Electron Configuration:
The number of protons in the nucleus and the arrangement of electrons influence the strength of metallic bonding (for metals) or intermolecular forces (for non-metals). Elements with more protons exert a stronger pull on electrons, leading to higher melting points. Electron configuration, specifically the number of valence electrons, plays a crucial role. Elements with half-filled or completely filled valence shells often exhibit higher melting points due to enhanced stability.
3. Type of Bonding:
The type of chemical bonding significantly affects melting point.
-
Metallic Bonding: Metals are characterized by a "sea" of delocalized electrons shared among positively charged metal ions. The strength of this metallic bond, influenced by factors like atomic size, electron configuration, and number of valence electrons, directly correlates with the melting point. Stronger metallic bonds translate to higher melting points.
-
Covalent Bonding: Covalent bonds, characterized by shared electron pairs, can result in high melting points, particularly in network covalent structures like diamond and silicon dioxide (SiO₂). These substances have strong, extensive networks of covalent bonds, requiring significant energy to break. However, discrete molecules held together by weaker intermolecular forces (van der Waals forces, hydrogen bonds) will generally have lower melting points.
-
Ionic Bonding: Ionic compounds, formed by electrostatic attraction between oppositely charged ions, typically exhibit high melting points. The strong electrostatic forces require considerable energy to overcome. However, the melting point is sensitive to the charge and size of the ions. Higher charges and smaller ions lead to stronger attractions and higher melting points.
4. Allotropes:
Elements that can exist in multiple structural forms (allotropes) will exhibit varying melting points depending on the allotrope. For instance, carbon exists as diamond (extremely high melting point) and graphite (relatively low melting point). This difference arises from the distinct bonding arrangements in each allotrope.
Trends Across Periods and Groups
Let's analyze the melting point trends across periods (rows) and groups (columns) of the periodic table.
Period Trends:
Moving across a period from left to right, the melting point generally increases initially, reaching a peak near the transition metals, and then decreases.
-
Initially Increasing Melting Points: In the early part of the period, the increase is primarily due to increasing metallic bonding strength as more valence electrons participate in the delocalized electron sea.
-
Peak Near Transition Metals: Transition metals often exhibit high melting points due to the involvement of d-electrons in metallic bonding, leading to stronger interactions. The multiple oxidation states possible for transition metals also contribute to the complexity of their bonding behavior.
-
Decreasing Melting Points: Towards the end of the period, the trend reverses. The elements become non-metals, forming weaker covalent bonds or existing as discrete molecules with weak intermolecular forces. These weaker forces require less energy to overcome during melting, resulting in lower melting points.
Group Trends:
The group (column) trends in melting point are more complex and depend on the specific group.
-
Group 1 (Alkali Metals): Melting points generally decrease down the group as atomic size increases and metallic bonding weakens.
-
Group 2 (Alkaline Earth Metals): Similar to Group 1, a decrease in melting point is observed down the group due to increasing atomic size. However, the melting points are generally higher than their alkali metal counterparts because of the stronger metallic bonding resulting from two valence electrons.
-
Group 17 (Halogens): The melting points increase down the group, primarily due to increasing strength of van der Waals forces as atomic size and number of electrons increase. Larger molecules have more surface area for interaction, resulting in stronger intermolecular forces and higher melting points.
-
Group 18 (Noble Gases): The melting points increase down the group due to increased van der Waals forces, similar to the halogens. However, these melting points remain extremely low due to the weak nature of these forces.
-
Transition Metals: The melting point trends within transition metal groups are less straightforward and influenced by various factors like electronic configuration, d-electron interactions, and crystal structure. No single consistent trend is universally applicable.
Exceptions and Irregularities
The periodic trends discussed above are generalizations, and numerous exceptions exist. These exceptions highlight the interplay of multiple factors influencing melting points.
-
Carbon (Diamond vs. Graphite): As mentioned earlier, the drastically different melting points of diamond and graphite illustrate the importance of allotropic forms.
-
Boron: Boron has an unusually high melting point due to its strong covalent network structure.
-
Silicon and Germanium: Similar to boron, silicon and germanium exhibit higher melting points than might be predicted based solely on their position in the periodic table due to their strong covalent network structures.
-
Some Transition Metals: Certain transition metals exhibit unexpected melting points, influenced by their specific electronic configurations and crystal structures.
Applications of Melting Point Knowledge
Understanding melting point trends has numerous applications:
-
Material Science: The knowledge of melting points is crucial in material selection and processing. High-melting-point materials are essential for applications demanding high-temperature stability, while low-melting-point materials are needed in soldering and other applications requiring low-temperature processing.
-
Geochemistry: Melting point data is used to understand the formation and evolution of rocks and minerals in the Earth's crust and mantle. The melting points of various minerals dictate their behavior at different temperatures and pressures.
-
Chemical Synthesis and Purification: Melting points are used to identify and purify chemical compounds. The melting point range is a characteristic property used for substance identification and assessment of purity.
-
Metallurgy: Understanding the melting points of metals and alloys is essential in metallurgical processes like casting, welding, and shaping metals.
Conclusion
The melting point of an element is a complex property governed by a combination of atomic size, atomic number, electron configuration, type of bonding, and allotropic forms. While general trends exist across the periodic table, significant exceptions highlight the intricate interplay of these factors. Understanding these trends and exceptions is vital across various scientific disciplines, particularly in material science, geochemistry, chemical synthesis, and metallurgy. Further research continues to refine our understanding of melting point behavior and its implications. The journey of exploring the intricacies of the periodic table's melting point trends continues, offering fascinating insights into the fundamental forces shaping the materials world around us.
Latest Posts
Latest Posts
-
Is Orange Juice A Mixture Or Pure Substance
Mar 24, 2025
-
Creating A Frequency Chart In Excel
Mar 24, 2025
-
How Does A Buffer Solution Resist A Change In Ph
Mar 24, 2025
-
Which Of The Following Are Chemical Reactions
Mar 24, 2025
-
Do Strong Acids Have High Or Low Ka
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Trend Of Melting Point In Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
