Two Or More Elements Chemically Combined
Muz Play
Mar 26, 2025 · 6 min read
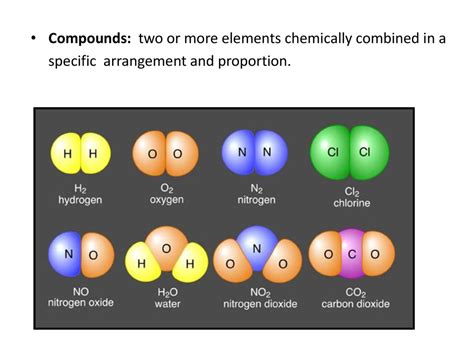
Table of Contents
Two or More Elements Chemically Combined: A Deep Dive into Compounds
When two or more elements chemically combine, they form a compound. This seemingly simple statement underpins the vast complexity of chemistry and the material world around us. Understanding how elements combine to create compounds is fundamental to grasping the properties of matter, from the air we breathe to the food we eat, and the technologies that shape our lives. This article will explore the fascinating world of compounds, delving into their formation, properties, and the diverse ways they impact our existence.
The Nature of Chemical Bonds: The Glue that Holds Compounds Together
The key to understanding compounds lies in understanding chemical bonds. These are the forces of attraction that hold atoms together in a molecule or crystal. Several types of chemical bonds exist, each with unique characteristics:
1. Ionic Bonds: The Electrostatic Attraction
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal, loses one or more electrons to become a positively charged cation, and another atom, usually a non-metal, gains those electrons to become a negatively charged anion. The strong coulombic force between these ions creates a stable ionic compound. A classic example is sodium chloride (NaCl), or table salt, where sodium (Na) loses an electron to become Na⁺ and chlorine (Cl) gains that electron to become Cl⁻.
Keywords: Ionic bond, cation, anion, electrostatic attraction, coulombic force, sodium chloride, NaCl.
2. Covalent Bonds: Sharing is Caring
In contrast to ionic bonds, covalent bonds involve the sharing of electrons between atoms. This is particularly common between non-metal atoms. The shared electrons are attracted to the nuclei of both atoms, creating a stable bond. The strength of a covalent bond depends on factors like the number of shared electron pairs and the electronegativity difference between the atoms. Examples include water (H₂O), where oxygen shares electrons with two hydrogen atoms, and methane (CH₄), where carbon shares electrons with four hydrogen atoms.
Keywords: Covalent bond, electron sharing, electronegativity, water, H₂O, methane, CH₄.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals. In these materials, the valence electrons are delocalized, forming a "sea" of electrons that surrounds positively charged metal ions. This sea of electrons allows for high electrical and thermal conductivity, malleability, and ductility, characteristic properties of metals.
Keywords: Metallic bond, delocalized electrons, electrical conductivity, thermal conductivity, malleability, ductility.
4. Hydrogen Bonds: A Special Case of Intermolecular Force
While not strictly a chemical bond in the same way as ionic, covalent, or metallic bonds, hydrogen bonds are crucial intermolecular forces that significantly affect the properties of many compounds. They occur between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Hydrogen bonds are responsible for the high boiling point of water and the structure of proteins and DNA.
Keywords: Hydrogen bond, intermolecular force, electronegative atom, boiling point, protein, DNA.
Properties of Compounds: A Diverse Landscape
The properties of a compound are vastly different from the properties of its constituent elements. This is because the chemical combination leads to a new substance with its own unique characteristics. These properties can be physical or chemical:
Physical Properties: Observable Characteristics
Physical properties are characteristics that can be observed or measured without changing the chemical composition of the compound. These include:
- Melting point: The temperature at which a solid turns into a liquid.
- Boiling point: The temperature at which a liquid turns into a gas.
- Density: The mass per unit volume.
- Solubility: The ability to dissolve in a solvent.
- Color: The appearance of the compound.
- Odor: The smell of the compound.
Chemical Properties: Reactivity and Transformations
Chemical properties describe how a compound reacts with other substances. These properties determine how a compound will behave in chemical reactions, including:
- Reactivity: How readily the compound undergoes chemical changes.
- Combustibility: The ability to burn in the presence of oxygen.
- Acidity/Basicity: The ability to donate or accept protons (H⁺ ions).
- Oxidation/Reduction: The ability to gain or lose electrons.
Types of Compounds: A Vast and Varied World
The sheer number and diversity of compounds are staggering. They can be broadly categorized based on their composition and structure:
1. Organic Compounds: The Carbon Backbone
Organic compounds contain carbon atoms bonded to other carbon atoms and/or hydrogen atoms, often with other elements like oxygen, nitrogen, sulfur, and phosphorus. The vast majority of organic compounds are based on the carbon atom's unique ability to form long chains and rings. Examples include hydrocarbons (like methane and ethane), alcohols (like ethanol), and carbohydrates (like glucose). The study of organic chemistry is crucial for understanding life itself, as living organisms are primarily composed of organic molecules.
Keywords: Organic compound, carbon, hydrocarbon, alcohol, carbohydrate, glucose, ethanol.
2. Inorganic Compounds: A Diverse Group
Inorganic compounds encompass all compounds that are not organic. This is a broad category, including ionic compounds (like salts), oxides (like water and carbon dioxide), acids (like hydrochloric acid and sulfuric acid), and bases (like sodium hydroxide and ammonia). Many inorganic compounds are essential components of rocks, minerals, and industrial materials.
Keywords: Inorganic compound, ionic compound, oxide, acid, base, salt.
3. Binary Compounds: Two-Element Simplicity
Binary compounds are the simplest type of compound, consisting of only two different elements. Examples include water (H₂O), sodium chloride (NaCl), and carbon dioxide (CO₂). Their properties are determined by the nature of the bond between the two elements and their relative electronegativities.
Keywords: Binary compound, water, H₂O, sodium chloride, NaCl, carbon dioxide, CO₂.
Applications of Compounds: Shaping Our World
Compounds play an indispensable role in nearly every aspect of modern life:
- Medicine: Many drugs and pharmaceuticals are organic compounds designed to interact with specific biological targets.
- Materials Science: Compounds are used to create a vast array of materials, from plastics and polymers to ceramics and metals.
- Agriculture: Fertilizers and pesticides are often complex compounds designed to enhance crop yields or control pests.
- Energy: Fossil fuels (compounds of carbon and hydrogen) are a major source of energy, while research into new energy technologies utilizes many different types of compounds.
- Food Science: Many food additives, preservatives, and flavorings are specific chemical compounds.
Conclusion: The Ongoing Exploration of Compounds
The study of compounds, their formation, properties, and applications, is a vast and ever-evolving field. New compounds are constantly being discovered and synthesized, leading to advancements in medicine, materials science, and countless other fields. The fundamental principles of chemical bonding and the diverse ways elements combine to form compounds remain at the heart of chemistry, providing the framework for understanding the material world and its potential. Further exploration into the intricacies of chemical structure and reactivity will undoubtedly continue to unlock new possibilities and innovations for the future. Understanding compounds is not merely an academic exercise; it's a key to understanding our world and shaping a better future.
Latest Posts
Latest Posts
-
What Are The Vertical Columns Called On A Periodic Table
Mar 29, 2025
-
Where Does Fermentation Take Place In A Cell
Mar 29, 2025
-
What Does A High Specific Heat Mean
Mar 29, 2025
-
Example Of Liquid Dissolved In Liquid
Mar 29, 2025
-
Write The Condensed Structure For Each Of These Skeletal Structures
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Two Or More Elements Chemically Combined . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
