Two Reactions Between A Grignard Reagent And A Carbonyl Compound
Muz Play
Apr 06, 2025 · 6 min read
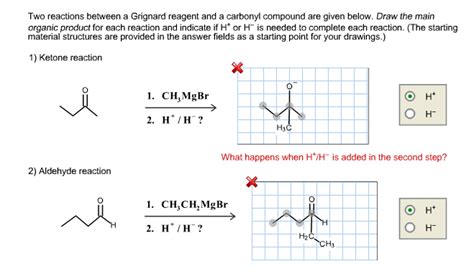
Table of Contents
Two Reactions Between a Grignard Reagent and a Carbonyl Compound: A Deep Dive
Grignard reagents, organomagnesium halides with the general formula RMgX (where R is an alkyl or aryl group and X is a halogen), are powerful tools in organic synthesis. Their exceptional nucleophilicity allows them to react readily with a wide range of electrophiles, most notably carbonyl compounds. This article delves into two crucial reactions between Grignard reagents and carbonyl compounds: the formation of secondary alcohols from aldehydes and tertiary alcohols from ketones. We'll explore the reaction mechanisms, synthetic applications, and limitations of these vital transformations.
Reaction 1: Grignard Reagent + Aldehyde → Secondary Alcohol
The reaction between a Grignard reagent and an aldehyde results in the formation of a secondary alcohol. This reaction proceeds via a nucleophilic addition mechanism. Let's break down the steps:
Mechanism:
-
Nucleophilic Attack: The Grignard reagent, acting as a strong nucleophile, attacks the electrophilic carbonyl carbon of the aldehyde. The electrons in the carbonyl pi bond shift to the oxygen atom, creating an alkoxide intermediate. This step is crucial and determines the stereochemistry of the product if the aldehyde is chiral.
-
Protonation: The alkoxide intermediate is then protonated by an acidic workup, typically using a dilute aqueous solution of an acid like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄). This protonation step converts the negatively charged oxygen into a hydroxyl group (-OH), yielding the secondary alcohol product.
Illustrative Example:
The reaction between methylmagnesium bromide (CH₃MgBr) and formaldehyde (HCHO) forms methanol (CH₃OH), a primary alcohol. However, if we use any other aldehyde, a secondary alcohol will be obtained. For example, the reaction between ethylmagnesium bromide (CH₃CH₂MgBr) and propanal (CH₃CH₂CHO) produces 3-pentanol (CH₃CH₂CH(OH)CH₂CH₃), a secondary alcohol:
CH₃CH₂CHO + CH₃CH₂MgBr → CH₃CH₂CH(OMgBr)CH₂CH₃ → CH₃CH₂CH(OH)CH₂CH₃
Synthetic Applications:
This reaction is invaluable for the synthesis of a wide array of secondary alcohols. By carefully choosing the Grignard reagent and the aldehyde, chemists can precisely control the structure of the resulting alcohol. This is particularly useful in the synthesis of complex molecules and pharmaceuticals where stereochemical control is essential. Consider the synthesis of chiral secondary alcohols: the use of enantiomerically pure aldehydes and Grignard reagents can lead to enantiomerically pure secondary alcohols, a key requirement in many drug molecules.
Limitations:
While highly versatile, this reaction has certain limitations:
-
Reactivity of the Aldehyde: Highly hindered aldehydes react more slowly or may not react at all. Steric hindrance around the carbonyl group can impede the nucleophilic attack by the Grignard reagent.
-
Side Reactions: Grignard reagents are highly reactive and can undergo side reactions, especially with other electrophilic functional groups present in the molecule. Careful consideration of the aldehyde's structure is needed to prevent these competing reactions.
-
Water Sensitivity: Grignard reagents are extremely sensitive to water and must be handled under anhydrous conditions. The presence of water can lead to the decomposition of the Grignard reagent before it can react with the aldehyde.
Reaction 2: Grignard Reagent + Ketone → Tertiary Alcohol
The reaction between a Grignard reagent and a ketone yields a tertiary alcohol. The mechanism closely resembles that of the aldehyde reaction, but the product differs due to the initial ketone structure.
Mechanism:
-
Nucleophilic Attack: Similar to the aldehyde reaction, the Grignard reagent attacks the electrophilic carbonyl carbon of the ketone. The electrons in the carbonyl pi bond again shift to the oxygen atom, resulting in a tertiary alkoxide intermediate. Note that the stereochemistry at this stage is crucial if the ketone is chiral.
-
Protonation: The alkoxide intermediate is protonated during the acidic workup (e.g., using dilute aqueous HCl), generating the tertiary alcohol.
Illustrative Example:
The reaction between phenylmagnesium bromide (C₆H₅MgBr) and acetophenone (C₆H₅COCH₃) produces a tertiary alcohol:
C₆H₅COCH₃ + C₆H₅MgBr → (C₆H₅)₂C(OMgBr)CH₃ → (C₆H₅)₂C(OH)CH₃
The product, 2,2-diphenylethanol, is a tertiary alcohol. Notice the formation of the tertiary carbon atom bearing the hydroxyl group.
Synthetic Applications:
This reaction is equally important for synthesizing tertiary alcohols, particularly those with complex structures. The ability to introduce different alkyl or aryl groups via the Grignard reagent offers considerable control over the final product's structure. This opens pathways for synthesizing tertiary alcohols with specific properties tailored for various applications. Furthermore, this reaction plays a key role in the synthesis of various natural products and pharmaceuticals containing tertiary alcohol functional groups.
Limitations:
Similar to the aldehyde reaction, limitations exist:
-
Steric Hindrance: Ketones with bulky substituents can hinder the nucleophilic attack by the Grignard reagent, slowing down the reaction rate or preventing it altogether.
-
Competing Reactions: The presence of other electrophilic functional groups in the ketone can lead to side reactions, complicating the synthesis and potentially lowering the yield of the desired tertiary alcohol.
-
Anhydrous Conditions: Maintaining anhydrous conditions throughout the reaction is crucial. Water will react with the Grignard reagent, hindering the desired reaction and potentially leading to unwanted byproducts.
Comparative Analysis of Aldehyde and Ketone Reactions
Both reactions, while similar mechanistically, lead to different alcohol types: secondary from aldehydes and tertiary from ketones. This difference stems from the initial carbonyl compound's structure. The aldehyde has one alkyl/aryl group attached to the carbonyl carbon, while the ketone has two. This affects the substitution pattern of the resulting alcohol.
| Feature | Aldehyde Reaction | Ketone Reaction |
|---|---|---|
| Grignard Reagent | RMgX | RMgX |
| Carbonyl Compound | Aldehyde (RCHO) | Ketone (R₂CO) |
| Product | Secondary Alcohol (R₂CHOH) | Tertiary Alcohol (R₃COH) |
| Steric Hindrance | Less significant initially, but can be affected by the aldehyde and Grignard reagent size | More significant due to two alkyl/aryl groups |
| Synthetic Utility | Synthesis of secondary alcohols | Synthesis of tertiary alcohols |
Conclusion
The reactions between Grignard reagents and carbonyl compounds (aldehydes and ketones) are fundamental reactions in organic chemistry, providing efficient pathways for synthesizing secondary and tertiary alcohols, respectively. Understanding the mechanism, synthetic applications, and limitations of these reactions is crucial for any organic chemist. By carefully selecting the appropriate Grignard reagent and carbonyl compound, and by employing suitable reaction conditions, chemists can achieve high yields and selectivity in the synthesis of a wide range of alcohol derivatives crucial for various applications including pharmaceuticals, agrochemicals, and materials science. The ability to control stereochemistry is especially valuable in the production of enantiomerically pure alcohols, which are essential building blocks in the synthesis of many biologically active compounds. Further exploration of these reactions and their modifications will undoubtedly contribute to significant advancements in organic synthesis.
Latest Posts
Latest Posts
-
How To Write An Equation Of A Vertical Line
Apr 07, 2025
-
What Is The Building Block Monomer For A Nucleic Acids
Apr 07, 2025
-
Where Is The Serous Membrane Located
Apr 07, 2025
-
Is A Colloid A Homogeneous Mixture
Apr 07, 2025
-
What Do Fats Steroids And Waxes Have In Common
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Two Reactions Between A Grignard Reagent And A Carbonyl Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
