Use Iupac Rules To Name The Following Alkane
Muz Play
Mar 26, 2025 · 6 min read
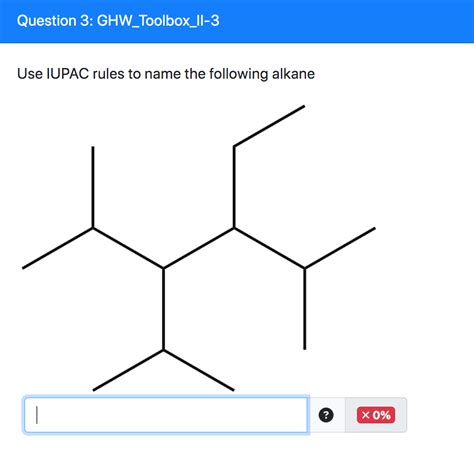
Table of Contents
Demystifying Alkane Nomenclature: A Comprehensive Guide Using IUPAC Rules
Naming organic compounds, especially alkanes, can seem daunting at first. However, with a systematic understanding of IUPAC (International Union of Pure and Applied Chemistry) rules, the process becomes straightforward and logical. This comprehensive guide will walk you through the process, providing numerous examples and clarifying potential ambiguities. We'll delve into the intricacies of identifying parent chains, numbering carbons, assigning substituents, and correctly applying prefixes and suffixes. By the end, you'll be confidently naming even complex alkane structures.
Understanding the Foundation: Alkanes and IUPAC
Alkanes are saturated hydrocarbons, meaning they contain only single carbon-carbon bonds and hydrogen atoms. Their general formula is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. IUPAC nomenclature provides a standardized system for naming organic compounds, ensuring consistent communication among chemists worldwide. This system is based on a set of rules that dictate how to identify and name the parent chain and any attached substituents.
The Core Rules of IUPAC Alkane Nomenclature
Before diving into complex examples, let's solidify the fundamental rules:
-
Identify the Longest Continuous Carbon Chain: This chain forms the basis of the parent alkane name. It's crucial to find the longest chain, even if it means it's not a straight line. The chain may be zig-zagged or folded.
-
Number the Carbon Atoms: Begin numbering the carbon atoms in the longest chain from the end that gives the lowest numbers to the substituents. If the substituents are equidistant from both ends, begin numbering from the end that gives the substituent with alphabetical priority the lower number.
-
Identify and Name the Substituents: Substituents are groups of atoms attached to the main carbon chain. Alkyl groups, derived from alkanes by removing one hydrogen atom, are the most common type. For example, removing a hydrogen from methane (CH<sub>4</sub>) yields a methyl group (CH<sub>3</sub>-), and removing a hydrogen from ethane (C<sub>2</sub>H<sub>6</sub>) yields an ethyl group (C<sub>2</sub>H<sub>5</sub>-).
-
Number the Substituents: Indicate the position of each substituent by the number of the carbon atom to which it is attached. If the same substituent appears multiple times, use prefixes like di-, tri-, tetra-, etc. to indicate the number of occurrences.
-
Arrange Substituents Alphabetically: When writing the full name, list the substituents alphabetically, ignoring prefixes like di-, tri-, etc. However, do consider the full alkyl group name (e.g., isopropyl before methyl).
-
Combine the Information: The complete name is formed by combining the numbers and names of the substituents, followed by the name of the parent alkane. Use hyphens to separate numbers from words and commas to separate numbers.
Working Through Examples: From Simple to Complex
Let's apply these rules to various alkanes, starting with simpler structures and progressing to more complex ones:
Example 1: A Simple Straight-Chain Alkane
Consider the alkane with the formula CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>.
- Longest chain: 5 carbon atoms.
- Parent alkane: Pentane.
- Substituents: None.
- Name: Pentane
Example 2: An Alkane with a Single Substituent
Consider the alkane: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>.
- Longest chain: 4 carbon atoms.
- Parent alkane: Butane.
- Substituents: One methyl group on carbon 2.
- Name: 2-Methylbutane
Example 3: An Alkane with Multiple Substituents
Consider the alkane: CH<sub>3</sub>CH(CH<sub>3</sub>)CH(CH<sub>3</sub>)CH<sub>3</sub>.
- Longest chain: 4 carbon atoms.
- Parent alkane: Butane.
- Substituents: Two methyl groups, one on carbon 2 and one on carbon 3.
- Name: 2,3-Dimethylbutane
Example 4: Prioritizing Alphabetical Order
Consider the alkane: CH<sub>3</sub>CH(CH<sub>2</sub>CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>.
- Longest chain: 4 carbon atoms.
- Parent alkane: Butane.
- Substituents: One ethyl group on carbon 2.
- Name: 2-Ethylbutane
Example 5: A More Complex Alkane
Consider a more complex structure: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH(C<sub>2</sub>H<sub>5</sub>)CH<sub>3</sub>
- Longest chain: 5 carbons.
- Parent Alkane: Pentane
- Substituents: A methyl group on carbon 2 and an ethyl group on carbon 4. Note that numbering from the right gives lower numbers to the substituents.
- Name: 2-Methyl-4-ethylpentane (Ethyl comes before methyl alphabetically).
Example 6: Dealing with Branched Substituents
Consider the alkane: CH<sub>3</sub>C(CH<sub>3</sub>)<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>.
- Longest chain: 4 carbons.
- Parent alkane: Butane
- Substituents: A tertiary-butyl group (or 1,1-dimethylethyl) on carbon 2. This requires understanding more complex alkyl group names.
- Name: 2,2-Dimethylbutane (Simpler to name in this instance, although formally it's 2-(1,1-dimethylethyl)propane.)
Example 7: Cyclic Alkanes
Cyclic alkanes (alkanes in a ring) also follow specific IUPAC rules:
- Prefix: "cyclo-" is added to the beginning of the parent alkane name.
- Numbering: Number the carbon atoms in the ring to give the lowest numbers to substituents. If there's more than one substituent, start numbering to give priority to the alphabetical order.
Consider cyclohexane with a methyl group and an ethyl group:
- Parent Alkane: Cyclohexane
- Substituents: 1-ethyl, 3-methyl
- Name: 1-Ethyl-3-methylcyclohexane.
Advanced Considerations and Common Pitfalls
While the basic principles are relatively straightforward, some situations require careful consideration:
-
Isomerism: Different arrangements of atoms can lead to isomers with identical molecular formulas but different structures and names. Precisely identifying the structure is critical for accurate naming.
-
Complex Substituents: Larger or more branched substituents require careful identification and naming using appropriate alkyl group names (e.g., isopropyl, sec-butyl, tert-butyl).
-
Ambiguous Numbering: In some cases, multiple numbering schemes might seem equally valid. The rules prioritize the lowest numbers possible, and if there are several equally valid lowest numbers, alphabetical ordering is used to resolve the ambiguity.
-
Stereochemistry: IUPAC nomenclature also incorporates stereochemical descriptors (e.g., R/ S, E/ Z) to indicate the spatial arrangement of atoms, but this is typically reserved for more advanced organic chemistry.
Conclusion: Mastering Alkane Nomenclature
Mastering IUPAC nomenclature for alkanes is a fundamental skill in organic chemistry. By following the systematic rules outlined above and practicing with numerous examples, you'll develop the confidence and proficiency to name even the most complex alkane structures accurately and efficiently. Remember to always start by identifying the longest carbon chain, then systematically number, identify, and name substituents following the alphabetical priority. Continuous practice is key to solidifying your understanding and building a strong foundation in organic chemistry. With consistent effort, you will become proficient in the art of alkane nomenclature.
Latest Posts
Latest Posts
-
What Is A Monomer Of Nucleic Acids Called
Mar 29, 2025
-
What Functional Groups Are In Aspirin
Mar 29, 2025
-
What Are The Elements In Group 18 Called
Mar 29, 2025
-
How Do You Make A Standard Curve
Mar 29, 2025
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Use Iupac Rules To Name The Following Alkane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
