What Functional Groups Are In Aspirin
Muz Play
Mar 29, 2025 · 6 min read
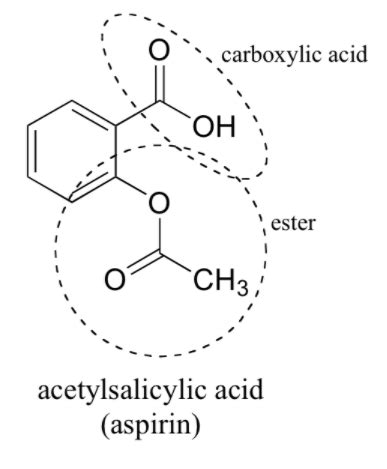
Table of Contents
What Functional Groups are in Aspirin? A Deep Dive into its Molecular Structure
Aspirin, a globally recognized and widely used medication, boasts a simple yet remarkably effective molecular structure. Understanding its functional groups is key to comprehending its pharmacological actions and properties. This detailed exploration delves into the specific functional groups present in aspirin, their impact on its characteristics, and the significance of these groups in its therapeutic efficacy.
The Chemical Structure of Aspirin: A Foundation for Understanding
Aspirin, also known chemically as acetylsalicylic acid, possesses a relatively straightforward molecular structure. Its chemical formula, C₉H₈O₄, hints at the presence of several oxygen-containing functional groups. These functional groups aren't merely components; they are the driving force behind aspirin's ability to act as an analgesic (pain reliever), antipyretic (fever reducer), and anti-inflammatory agent.
Image: [Here you would insert an image of the aspirin molecular structure. Since I cannot directly insert images, imagine a clear, well-labeled diagram showing the molecule with its functional groups clearly highlighted. You could easily find suitable images through a Google image search for "aspirin molecular structure." Label the carboxylic acid, ester, and aromatic ring.]
Identifying the Key Functional Groups in Aspirin
Aspirin's pharmacological activity stems directly from its three key functional groups:
1. Carboxylic Acid Group (-COOH): The Heart of Aspirin's Acidity
The carboxylic acid group is arguably the most significant functional group in aspirin. Located at one end of the molecule, this -COOH group is responsible for aspirin's acidic nature. The hydrogen atom in the -OH group is relatively easily lost as a proton (H+), making aspirin a weak acid. This acidity plays a crucial role in:
- Solubility: The carboxylic acid group influences aspirin's solubility in different environments. While relatively insoluble in water at neutral pH, its solubility increases in alkaline solutions due to the formation of carboxylate ions (-COO⁻). This property is crucial for formulation and absorption in the body.
- Pharmacological Action: The acidity is linked to aspirin's mechanism of action, particularly its anti-inflammatory properties. The ability to donate a proton contributes to its interactions with target proteins involved in the inflammatory response.
- Drug Stability: The stability of aspirin is influenced by the carboxylic acid group. Under certain conditions, it can be susceptible to hydrolysis, especially in the presence of moisture, breaking down into salicylic acid and acetic acid. This instability is a consideration in its formulation and storage.
2. Ester Group (-COO-): A Crucial Link in the Aspirin Molecule
The ester group, -COO-, is formed by the reaction of a carboxylic acid (in this case, salicylic acid) and an alcohol (acetic acid). This group is the distinguishing feature differentiating aspirin from its precursor, salicylic acid. The ester linkage is the result of acetylation of salicylic acid, a pivotal step in aspirin synthesis. Its presence significantly alters the properties of the molecule:
- Reduced Gastric Irritation: Salicylic acid, the parent compound, is considerably more irritating to the stomach lining than aspirin. The ester group in aspirin modifies the molecule's acidity and reduces its direct interaction with the stomach's mucous membrane, lessening the likelihood of gastrointestinal upset. This is a crucial improvement in the drug's tolerability profile.
- Enhanced Bioavailability: The ester bond is crucial for aspirin's absorption and metabolism. Once ingested, aspirin is hydrolyzed back to salicylic acid in the body, releasing the active moiety. The ester linkage ensures the drug reaches the target sites before being fully broken down. This contributes to its bioavailability and effectiveness.
- Modified Pharmacokinetics: The presence of the ester group changes aspirin's pharmacokinetic profile, influencing its absorption, distribution, metabolism, and excretion within the body.
3. Aromatic Benzene Ring: Providing Structural Stability and Lipophilicity
The benzene ring is a crucial component of aspirin's structure. It's a six-carbon ring with alternating single and double bonds, conferring a high degree of stability and rigidity to the molecule. The aromatic ring plays several vital roles:
- Structural Rigidity: The planar structure contributes to the overall shape and conformation of the molecule, influencing its interactions with biological receptors. This inherent rigidity contributes to aspirin's selectivity for certain target sites.
- Lipophilicity: The benzene ring is hydrophobic (water-repelling), rendering aspirin relatively lipophilic (fat-soluble). This lipophilicity facilitates its absorption from the gastrointestinal tract and its distribution to various tissues in the body.
- Metabolic Stability: The aromatic ring offers some protection against rapid metabolism, extending the duration of action in the body. However, it's worth noting that the molecule undergoes extensive metabolism, primarily in the liver.
The Interplay of Functional Groups: A Synergistic Effect
The functional groups within the aspirin molecule don't act in isolation; instead, they demonstrate a synergistic effect. The interaction between the carboxylic acid, ester, and aromatic ring creates a unique combination of properties:
- Balance of Acidity and Lipophilicity: The interplay between the carboxylic acid's acidity and the aromatic ring's lipophilicity results in a molecule that is sufficiently soluble for absorption yet lipophilic enough to penetrate cell membranes and interact with its target proteins. This balance is vital for its effectiveness.
- Metabolic Pathways: The ester group dictates the initial metabolic pathways aspirin takes, resulting in the release of salicylic acid. This released salicylic acid, which itself possesses therapeutic activity, can further undergo other metabolic transformations.
- Target Site Interactions: The combined properties of these functional groups enable aspirin to interact with various enzymes and proteins involved in pain, fever, and inflammation. The precise interactions and mechanism of action are complex and subject to ongoing research.
Aspirin's Therapeutic Applications: A Consequence of its Functional Groups
The presence and interplay of these functional groups directly relate to aspirin's wide range of therapeutic applications:
- Analgesic (Pain Relief): Aspirin inhibits the production of prostaglandins, inflammatory mediators that contribute to pain sensation. This action is primarily attributed to the salicylic acid released after ester hydrolysis.
- Antipyretic (Fever Reduction): Similar to its analgesic effect, aspirin's antipyretic action stems from its ability to inhibit prostaglandin synthesis in the hypothalamus, the body's temperature-regulating center.
- Anti-inflammatory: Aspirin's ability to reduce inflammation is also related to its prostaglandin-inhibiting action. Prostaglandins are crucial mediators in the inflammatory cascade, and aspirin's suppression of their production helps reduce swelling, redness, and pain.
- Antiplatelet (Blood Thinning): This action is particularly relevant in cardiovascular disease management. Aspirin irreversibly inhibits platelet aggregation by inhibiting cyclooxygenase (COX), an enzyme involved in thromboxane production. Thromboxane is a crucial mediator in blood clot formation. This property is responsible for aspirin's use in preventing heart attacks and strokes.
Conclusion: The Importance of Understanding Aspirin's Functional Groups
Understanding the specific functional groups in aspirin – the carboxylic acid, ester, and aromatic benzene ring – provides a comprehensive understanding of its properties, pharmacological actions, and therapeutic efficacy. The unique combination and interaction of these groups create a molecule that is both effective and widely used for a range of health conditions. Further research into these functional groups and their interactions continues to refine our understanding of aspirin's mechanism of action and potential applications. This detailed analysis underscores the importance of understanding chemical structure and functionality in the development and application of effective medications.
Latest Posts
Latest Posts
-
Does A Trout Have Upright Erect Posture
Apr 01, 2025
-
What Holds The Hydrogen Atoms To The Oxygen Atom
Apr 01, 2025
-
Example Of Solid Dissolved In Liquid
Apr 01, 2025
-
What Is A Model In Biology
Apr 01, 2025
-
What Is A Normal Constituent Of Urine
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Functional Groups Are In Aspirin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
