Valence Bond Theory Vs Molecular Orbital Theory
Muz Play
Mar 22, 2025 · 6 min read
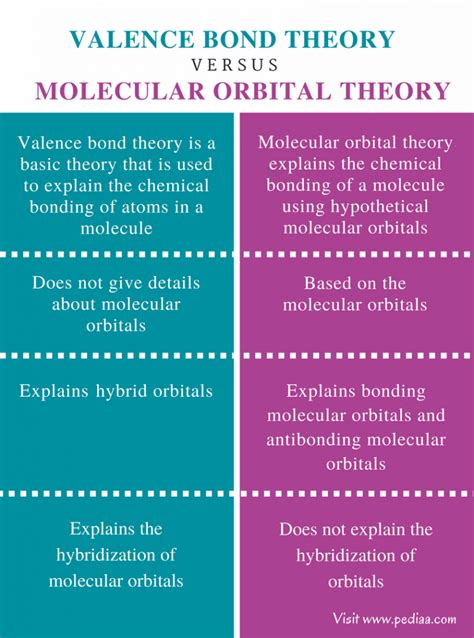
Table of Contents
Valence Bond Theory vs. Molecular Orbital Theory: A Comprehensive Comparison
Understanding how atoms bond together to form molecules is fundamental to chemistry. Two dominant theories explain this phenomenon: Valence Bond (VB) theory and Molecular Orbital (MO) theory. While both aim to describe molecular structure and bonding, they approach the problem from different perspectives, leading to varying strengths and weaknesses in their predictive capabilities. This article delves into a detailed comparison of VB theory and MO theory, highlighting their core concepts, advantages, disadvantages, and applications.
Valence Bond Theory: A Localized Approach
Valence Bond (VB) theory, developed primarily by Linus Pauling, adopts a localized perspective on bonding. It posits that a covalent bond forms when two atomic orbitals, each containing a single electron, overlap to share an electron pair. This shared electron pair is localized between the two bonded atoms. The greater the overlap, the stronger the bond.
Key Concepts of VB Theory:
- Atomic Orbitals: VB theory starts with the atomic orbitals of individual atoms, such as s, p, d, and f orbitals.
- Orbital Overlap: Bond formation occurs through the overlap of atomic orbitals. The extent of overlap determines bond strength. Greater overlap generally leads to a stronger bond.
- Hybrid Orbitals: To explain the geometry and bonding in molecules like methane (CH₄), VB theory introduces the concept of hybrid orbitals. These are formed by mixing atomic orbitals of similar energy to create new orbitals with different shapes and orientations, better suited for bonding. Examples include sp, sp², and sp³ hybridization.
- Sigma (σ) and Pi (π) Bonds: VB theory distinguishes between sigma (σ) and pi (π) bonds. Sigma bonds are formed by head-on overlap of atomic orbitals, while pi bonds result from sideways overlap. Sigma bonds are stronger than pi bonds.
- Resonance: For molecules with multiple Lewis structures (resonance structures), VB theory describes the molecule as a resonance hybrid, a weighted average of the contributing structures. This accounts for the delocalization of electrons.
Advantages of VB Theory:
- Intuitive and Easy to Visualize: The concept of overlapping atomic orbitals is relatively easy to grasp and visualize, making it an accessible model for beginners.
- Explains Molecular Geometry Well: VB theory, especially with the inclusion of hybridization, effectively explains the observed geometries of many molecules.
- Predicts Bond Strengths Relatively Accurately: The degree of orbital overlap provides a reasonable estimate of bond strength.
Disadvantages of VB Theory:
- Limited Applicability to Complex Molecules: VB theory becomes increasingly complex and difficult to apply to larger and more complex molecules.
- Difficulty in Explaining Magnetic Properties: VB theory struggles to explain the magnetic properties of some molecules, such as oxygen (O₂), which is paramagnetic despite having a seemingly complete electron pairing in its Lewis structure.
- Ignores Electron Delocalization in Some Cases: Although resonance addresses some instances of delocalization, it doesn't fully capture the extent of electron delocalization in certain molecules.
Molecular Orbital Theory: A Delocalized Approach
Molecular Orbital (MO) theory offers a more sophisticated and delocalized perspective on bonding. It considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule. Electrons are not confined to individual bonds but occupy molecular orbitals that can extend over the entire molecular framework.
Key Concepts of MO Theory:
- Linear Combination of Atomic Orbitals (LCAO): MO theory uses the LCAO method to combine atomic orbitals into molecular orbitals. The number of molecular orbitals formed equals the number of atomic orbitals combined.
- Bonding and Antibonding Molecular Orbitals: The combination of atomic orbitals leads to the formation of both bonding and antibonding molecular orbitals. Bonding orbitals are lower in energy than the constituent atomic orbitals and promote bonding, while antibonding orbitals are higher in energy and weaken bonding.
- Electron Configuration: Electrons fill the molecular orbitals according to the Aufbau principle and Hund's rule, similar to filling atomic orbitals. The electron configuration determines the overall bond order and magnetic properties.
- Bond Order: The bond order is calculated as half the difference between the number of electrons in bonding and antibonding molecular orbitals. It indicates the strength of the bond. A higher bond order generally signifies a stronger bond.
- Delocalization: MO theory naturally incorporates electron delocalization, providing a more accurate representation of electron distribution in molecules with conjugated pi systems.
Advantages of MO Theory:
- Superior in Describing Magnetic Properties: MO theory accurately predicts the magnetic properties of molecules, including those with unpaired electrons, such as oxygen (O₂).
- Handles Delocalization Effectively: MO theory naturally accounts for electron delocalization, providing a more realistic picture of electron distribution in conjugated systems.
- Applicable to Complex Molecules: MO theory is more readily adaptable to complex molecules and systems, although calculations can become computationally demanding.
- Provides a Quantitative Measure of Bond Strength: The bond order provides a quantitative measure of bond strength, offering greater predictive power.
Disadvantages of MO Theory:
- Less Intuitive and Harder to Visualize: The concept of delocalized molecular orbitals can be less intuitive and challenging to visualize than the localized orbitals of VB theory.
- Computationally Intensive for Larger Molecules: Performing MO calculations for larger molecules can become computationally expensive and require sophisticated software.
- Can Be Abstract for Beginners: The mathematical formalism behind MO theory can be daunting for those without a strong background in quantum mechanics.
Comparing VB and MO Theories: A Head-to-Head Analysis
| Feature | Valence Bond Theory | Molecular Orbital Theory |
|---|---|---|
| Approach | Localized | Delocalized |
| Bond Formation | Overlap of atomic orbitals | Combination of atomic orbitals into molecular orbitals |
| Electron Location | Localized between bonded atoms | Delocalized across the molecule |
| Geometry | Explains geometry well using hybridization | Explains geometry but requires more complex calculations |
| Bond Strength | Estimates bond strength based on overlap | Provides quantitative bond order |
| Magnetic Properties | Struggles with molecules having unpaired electrons | Accurately predicts magnetic properties |
| Delocalization | Addresses some delocalization through resonance | Naturally incorporates electron delocalization |
| Complexity | Simpler for small molecules, complex for larger ones | More complex, but more versatile for larger molecules |
| Computational Cost | Relatively low | Can be high for larger molecules |
Applications of VB and MO Theories
Both VB and MO theories find applications in various areas of chemistry:
- VB Theory: Primarily used in introductory organic chemistry courses to understand molecular geometry, bonding in organic molecules, and resonance structures.
- MO Theory: Widely used in advanced inorganic chemistry, physical chemistry, and computational chemistry to study the electronic structure, bonding, and reactivity of molecules, particularly those with conjugated pi systems or transition metal complexes. It's essential for understanding spectroscopy, catalysis, and materials science.
Conclusion: Choosing the Right Theory
The choice between VB and MO theory often depends on the specific problem being addressed and the level of detail required. VB theory provides a simpler and more intuitive model for understanding the basic principles of bonding in many common molecules. However, MO theory offers a more complete and accurate description of bonding, especially in complex molecules and systems where electron delocalization plays a significant role. In practice, chemists often employ a combination of both theories, utilizing the strengths of each to gain a comprehensive understanding of molecular structure and bonding. The increasing power of computational chemistry allows for more accurate MO calculations, making this theory increasingly prevalent in research and applications. Ultimately, both theories represent valuable tools in the chemist's arsenal for understanding the intricate world of molecular interactions.
Latest Posts
Latest Posts
-
How Is The Circulatory System Similar To A Road And Highway System
Mar 23, 2025
-
Diels Alder Reaction With Maleic Anhydride
Mar 23, 2025
-
Center Of Mass Of A Hemisphere
Mar 23, 2025
-
Organic Chemistry Substitution And Elimination Reactions
Mar 23, 2025
-
Arteries Of Lower Limb Flow Chart
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Valence Bond Theory Vs Molecular Orbital Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
