Diels Alder Reaction With Maleic Anhydride
Muz Play
Mar 23, 2025 · 5 min read
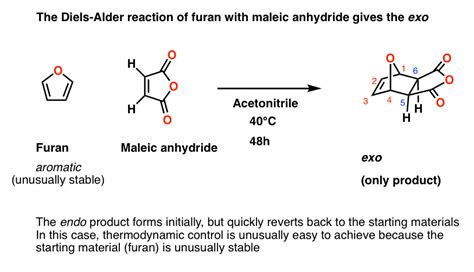
Table of Contents
The Diels-Alder Reaction with Maleic Anhydride: A Comprehensive Guide
The Diels-Alder reaction, a cornerstone of organic chemistry, stands as a powerful tool for constructing six-membered rings. Its versatility and predictable regio- and stereochemistry make it invaluable in both academic research and industrial applications. This article delves into the specifics of the Diels-Alder reaction utilizing maleic anhydride as the dienophile, exploring its mechanism, synthetic applications, and limitations.
Understanding the Diels-Alder Reaction
The Diels-Alder reaction is a [4+2] cycloaddition, meaning a four-carbon atom component (the diene) reacts with a two-carbon atom component (the dienophile) to form a six-membered ring. This reaction proceeds through a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously in a single step. This concerted nature dictates the stereochemistry of the product.
Key Characteristics:
- Concerted Mechanism: This is a crucial feature, leading to high stereospecificity. The relative stereochemistry of the diene and dienophile is preserved in the product.
- Stereospecificity: cis dienophiles yield cis products, and trans dienophiles yield trans products. This is a powerful tool for controlling stereochemistry in synthesis.
- Regioselectivity: The reaction often exhibits regioselectivity, favoring the formation of one regioisomer over another, depending on the substituents on the diene and dienophile.
- Pericyclic Reaction: The Diels-Alder reaction falls under the umbrella of pericyclic reactions, governed by orbital symmetry considerations. This means the HOMO (highest occupied molecular orbital) of the diene interacts with the LUMO (lowest unoccupied molecular orbital) of the dienophile, and vice-versa.
Maleic Anhydride: A Versatile Dienophile
Maleic anhydride, a cyclic anhydride with a carbon-carbon double bond, serves as an excellent dienophile in the Diels-Alder reaction. Its electron-withdrawing carbonyl groups significantly lower the energy of its LUMO, making it highly reactive towards dienes. This enhanced reactivity is crucial for achieving high yields and faster reaction rates.
Advantages of Using Maleic Anhydride:
- High Reactivity: The electron-withdrawing nature of the carbonyl groups increases the dienophile's reactivity.
- Readily Available: Maleic anhydride is inexpensive and readily available commercially.
- Crystalline Solid: Its solid nature simplifies handling and purification compared to liquid dienophiles.
- Versatile Product: The resulting anhydride group in the product offers various functionalization possibilities.
Mechanism of the Diels-Alder Reaction with Maleic Anhydride
The reaction mechanism involves the concerted [4+2] cycloaddition of the diene and maleic anhydride. The HOMO of the diene overlaps with the LUMO of maleic anhydride, forming new sigma bonds between the carbons. This process happens simultaneously, with no intermediate formation.
Step-by-Step Visualization:
- Orbital Overlap: The HOMO of the diene (typically a conjugated diene) interacts with the LUMO of maleic anhydride.
- Simultaneous Bond Formation: Two new sigma bonds form between the diene and dienophile carbons.
- Six-Membered Ring Formation: A cyclohexene derivative is formed, incorporating the anhydride moiety.
- Stereochemical Outcome: The cis geometry of the maleic anhydride is retained in the product.
Illustrative Example:
The reaction of 1,3-butadiene with maleic anhydride yields cis-norbornene-5,6-dicarboxylic anhydride:
[Insert image of 1,3-butadiene reacting with maleic anhydride to form cis-norbornene-5,6-dicarboxylic anhydride]
Factors Affecting the Diels-Alder Reaction with Maleic Anhydride
Several factors can significantly influence the outcome of the Diels-Alder reaction with maleic anhydride, including:
1. Diene Structure:
- Conjugation: A conjugated diene is essential for the reaction.
- Substituents: Electron-donating groups on the diene increase reactivity, while electron-withdrawing groups decrease it.
- Steric Hindrance: Bulky substituents can hinder the reaction and affect regioselectivity.
2. Dienophile Structure:
- Electron-Withdrawing Groups: As mentioned, electron-withdrawing groups on the dienophile enhance reactivity.
- Steric Hindrance: Similar to dienes, bulky substituents can impede the reaction.
3. Reaction Conditions:
- Temperature: Higher temperatures generally favor the reaction, but excessive heat can lead to side reactions.
- Solvent: The choice of solvent can influence the reaction rate and selectivity. A polar aprotic solvent like dichloromethane is often employed.
- Pressure: High pressure can accelerate the reaction.
Synthetic Applications of the Diels-Alder Reaction with Maleic Anhydride
The Diels-Alder reaction using maleic anhydride boasts a wide array of applications in organic synthesis:
1. Synthesis of Natural Products: Numerous natural products containing the bicyclic [2.2.1] framework are synthesized using this reaction as a key step.
2. Polymer Chemistry: The reaction is used to create polymers with specific properties, utilizing maleic anhydride as a monomer.
3. Medicinal Chemistry: The reaction is employed in the synthesis of various pharmaceuticals and drug candidates. The anhydride group offers opportunities for further functionalization to introduce desired pharmacophores.
4. Material Science: Maleic anhydride-derived Diels-Alder adducts are used in the creation of advanced materials with tailored properties, including strength, flexibility, and thermal stability.
Limitations of the Diels-Alder Reaction with Maleic Anhydride
Despite its versatility, the Diels-Alder reaction with maleic anhydride faces some limitations:
1. Regioselectivity Issues: In some cases, the reaction may lack regioselectivity, leading to mixtures of regioisomers. Careful selection of dienes and reaction conditions can help mitigate this.
2. Steric Hindrance: Bulky substituents on the diene or dienophile can significantly slow the reaction or prevent it altogether.
3. Reactivity of Dienes: Not all dienes are equally reactive with maleic anhydride; some require specific conditions or catalysts to achieve a reasonable yield.
Advanced Techniques and Modifications
Several techniques can improve the efficiency and selectivity of the Diels-Alder reaction with maleic anhydride:
1. Lewis Acid Catalysis: Lewis acids such as aluminum chloride or boron trifluoride can significantly accelerate the reaction and enhance regio- and stereoselectivity.
2. High-Pressure Reactions: Performing the reaction under high pressure increases reaction rates.
3. Microwave Irradiation: Microwave heating can substantially reduce reaction times.
4. Ultrasound-Assisted Reactions: Ultrasound can enhance mass transfer and promote faster reactions.
Conclusion
The Diels-Alder reaction with maleic anhydride is a powerful and versatile tool for organic synthesis, offering a straightforward and efficient method for constructing six-membered rings. Understanding the reaction mechanism, influencing factors, and various modifications allows chemists to effectively utilize this reaction in diverse synthetic endeavors, from the synthesis of complex natural products to the development of advanced materials. Its continued relevance in modern chemistry underscores its importance as a fundamental reaction in the organic chemist's toolkit. Further exploration of catalyst design and reaction conditions will undoubtedly expand the scope and applications of this transformative reaction.
Latest Posts
Latest Posts
-
Where Are The Neutrons Located In The Atom
Mar 24, 2025
-
Acids Release Which Type Of Ion In Water
Mar 24, 2025
-
What Is The Rectangular Coordinate System
Mar 24, 2025
-
Co Lewis Structure With Formal Charges
Mar 24, 2025
-
A Bunch Of Amino Acids Attached Together Is Called A
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Diels Alder Reaction With Maleic Anhydride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
