Co Lewis Structure With Formal Charges
Muz Play
Mar 24, 2025 · 6 min read
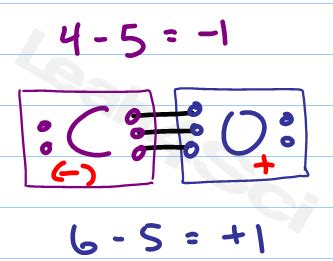
Table of Contents
Co Lewis Structure with Formal Charges: A Comprehensive Guide
Understanding the Lewis structure of carbon monoxide (CO) and its formal charges is crucial for grasping its bonding and reactivity. This comprehensive guide will delve into the intricacies of drawing the Lewis structure, calculating formal charges, and interpreting the results within the context of CO's unique properties. We'll also explore resonance structures and their contributions to the overall bonding picture.
Understanding Lewis Structures
A Lewis structure, also known as an electron dot structure, is a visual representation of the valence electrons in a molecule. It shows how atoms are bonded together and how the valence electrons are distributed. The structure provides insights into the molecule's geometry, polarity, and reactivity. Key elements of a Lewis structure include:
- Valence electrons: The outermost electrons of an atom, which participate in chemical bonding.
- Bonds: Lines representing shared electron pairs between atoms (covalent bonds).
- Lone pairs: Pairs of valence electrons not involved in bonding.
Drawing the Lewis Structure of Carbon Monoxide (CO)
Carbon (C) has four valence electrons, and oxygen (O) has six. Therefore, the total number of valence electrons in CO is 10 (4 + 6). To draw the Lewis structure:
- Identify the central atom: In CO, both atoms have similar electronegativity; however, carbon is often placed at the center by convention (though the final structure will be the same regardless).
- Connect the atoms with single bonds: Place a single bond (one line) between the carbon and oxygen atoms, using two of the ten valence electrons.
- Distribute the remaining electrons: Assign the remaining eight electrons (10 - 2 = 8) to the atoms to satisfy the octet rule (eight electrons surrounding each atom, except for hydrogen which follows the duet rule). Start by filling the octet of the more electronegative atom (oxygen), followed by carbon.
The initial structure would look like this:
:C-O:
However, this structure only uses 8 electrons, leaving two unused. This leads to an incomplete octet for both Carbon and Oxygen. To solve this, we need to utilize the remaining electrons and form a triple bond:
:C≡O:
This structure satisfies the octet rule for both carbon and oxygen. Each atom has eight electrons in its valence shell: oxygen has two lone pairs and three shared pairs, and carbon has one shared pair and three shared pairs.
Calculating Formal Charges
Formal charge helps to determine the best Lewis structure representation of a molecule. It's the hypothetical charge assigned to an atom in a molecule, assuming equal sharing of electrons in a covalent bond. The formula is:
Formal Charge = (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons)
Let's calculate the formal charges for both carbon and oxygen in the CO triple-bonded structure:
For Carbon:
- Valence electrons = 4
- Non-bonding electrons = 0
- Bonding electrons = 6
Formal Charge (C) = 4 - 0 - (1/2 * 6) = 1
For Oxygen:
- Valence electrons = 6
- Non-bonding electrons = 4
- Bonding electrons = 6
Formal Charge (O) = 6 - 4 - (1/2 * 6) = -1
Therefore, the formal charges for the most stable Lewis structure of CO are +1 for carbon and -1 for oxygen. This structure reflects the polar nature of the molecule, with oxygen being more electronegative and having a partial negative charge.
Resonance Structures in CO
While the triple-bonded structure is the most significant contributor, other resonance structures can be considered, although they are less significant. These alternative structures involve different arrangements of electrons but still maintain the same overall molecular formula. However, they are less energetically favorable and contribute less to the actual molecular structure. The main resonance structure, as determined by formal charge calculations, remains the triple bond structure.
The Importance of Formal Charges
Formal charges are crucial for several reasons:
- Predicting stability: Structures with formal charges closer to zero are generally more stable.
- Determining polarity: Formal charges indicate the distribution of electron density within the molecule, helping to predict its polarity.
- Understanding reactivity: Formal charges can help predict where a molecule is likely to react.
Other Lewis Structures and Their Instability
Let's briefly consider some other possible Lewis structures and why they are less favorable:
- A structure with a single bond: This would lead to incomplete octets for both atoms and very high formal charges, making it highly unstable.
- A structure with a double bond: This would give slightly lower formal charges than the triple bond structure but still wouldn’t be the most stable arrangement.
Conclusion: The Dominance of the Triple Bond Structure
Through careful analysis of Lewis structure construction and formal charge calculations, we find the triple-bonded structure with formal charges of +1 on carbon and -1 on oxygen to be the most plausible and energetically favorable Lewis structure of CO. This structure adequately explains the observed properties of carbon monoxide, such as its high bond order and polar nature. While other resonance structures may exist, their contribution is significantly less compared to the dominant triple-bonded structure. Understanding these concepts is fundamental for predicting and interpreting the reactivity and chemical behavior of carbon monoxide.
Beyond Formal Charges: Understanding the Bonding in CO
While formal charges provide a useful framework, they don't fully capture the complexity of bonding in CO. The triple bond signifies a strong bond, but the significant difference in electronegativity between carbon and oxygen leads to a polar covalent bond, with a partial positive charge on carbon and a partial negative charge on oxygen. Molecular orbital theory offers a more nuanced understanding of the bonding, showing the overlap of atomic orbitals to form bonding and antibonding molecular orbitals.
Applications and Significance of CO
Understanding the Lewis structure and bonding in CO is not just an academic exercise; it has significant practical applications. CO is a crucial molecule in various fields:
- Industrial Chemistry: It's a vital building block in the synthesis of many organic compounds, including methanol and acetic acid.
- Fuel Technology: It's a component of syngas (synthesis gas), a mixture used as a fuel source.
- Metallurgy: It plays a significant role in various metallurgical processes.
- Medical Applications: While toxic at high levels, controlled use of CO is being explored in targeted therapeutic applications.
This understanding of the CO molecule, starting from its Lewis structure, formal charges, and extending to a more complete understanding of its bonding, forms the basis for appreciating its role across a wide range of scientific and industrial applications. Furthermore, applying the principles discussed here will help in analysing the structure and properties of other molecules. The ability to accurately predict the most stable Lewis structure and calculate formal charges is an essential skill for anyone studying chemistry.
Latest Posts
Latest Posts
-
Coefficient Of Thermal Expansion Of Steel
Mar 28, 2025
-
Period 6 On The Periodic Table
Mar 28, 2025
-
The Feminization Of Poverty Refers To
Mar 28, 2025
-
What Is A Organic Sedimentary Rock
Mar 28, 2025
-
What Are Factors In An Experiment
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Co Lewis Structure With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
