Period 6 On The Periodic Table
Muz Play
Mar 28, 2025 · 6 min read
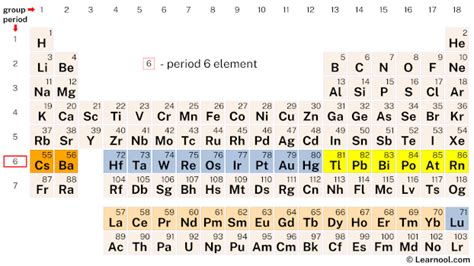
Table of Contents
Period 6: A Deep Dive into the Sixth Row of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Period 6, the sixth row of this iconic table, presents a fascinating array of elements, showcasing a unique blend of properties and behaviors. This comprehensive exploration delves into the characteristics, trends, and applications of Period 6 elements, offering a detailed understanding of this crucial section of the periodic table.
The Elements of Period 6: A Diverse Group
Period 6 houses 32 elements, ranging from the relatively unreactive lanthanides to the dense and radioactive actinides. This row marks the introduction of the f-block elements, significantly expanding the variety of chemical behavior observed. Let's break down the elements within Period 6, categorized for clarity:
The Transition Metals: A Spectrum of Properties
The transition metals of Period 6 (elements 72-80) demonstrate the characteristic properties of their group: variable oxidation states, colored compounds, and catalytic activity. These elements, including Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium (Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au), and Mercury (Hg), find diverse applications in various industries.
-
Tungsten (W): Known for its incredibly high melting point, tungsten is crucial in the manufacturing of light bulb filaments, high-speed steel alloys, and other high-temperature applications. Its strength and resistance to deformation make it invaluable in demanding environments.
-
Platinum (Pt): A highly valued noble metal, platinum is prized for its resistance to corrosion and its catalytic properties. It's used extensively in catalytic converters in automobiles, jewelry, and various chemical processes.
-
Gold (Au): A timeless symbol of wealth and prestige, gold's resistance to corrosion, malleability, and ductility make it ideal for coinage, jewelry, and electronics. Its unique properties also make it crucial in certain medical applications.
-
Mercury (Hg): The only liquid metal at room temperature, mercury has a rich history, but its toxicity limits its current applications significantly. Its use in thermometers and barometers is declining due to environmental concerns.
The Lanthanides: The Inner Transition Metals
The lanthanides (elements 57-71) are a series of 15 chemically similar elements found in Period 6. These elements, also known as the rare earth elements, share similar chemical properties due to the gradual filling of the 4f electron shell. Their properties are primarily dictated by their ionic radii and oxidation states. This chemical similarity makes their separation a challenging undertaking, often requiring advanced techniques. Despite their name, "rare earth," these elements are not particularly rare in the Earth's crust but are often dispersed making extraction difficult and costly.
- Applications of Lanthanides: Lanthanides are increasingly essential in various high-tech applications:
- Cerium (Ce): Used in self-cleaning ovens, catalytic converters, and some glass polishing compounds.
- Neodymium (Nd): Crucial component in powerful permanent magnets found in electric motors, wind turbines, and audio equipment.
- Europium (Eu): Used in red phosphors in color televisions and fluorescent lights.
- Yttrium (Y): A key component in high-temperature superconductors and certain lasers.
The Actinides: Radioactive and Reactive
The actinides (elements 89-103) are another series of inner transition metals. Unlike the lanthanides, most actinides are radioactive and exhibit a wider range of oxidation states. This radioactivity presents both challenges and opportunities. Their applications are often limited by their radioactivity and short half-lives but find crucial niches in various fields.
-
Uranium (U): Perhaps the most well-known actinide, uranium is a vital element in nuclear reactors, serving as fuel for generating electricity. Its use is tightly regulated due to the potential risks associated with nuclear materials.
-
Plutonium (Pu): Another critical element in nuclear technology, plutonium is also used in nuclear weapons due to its high fissile properties. Its radioactivity necessitates extremely careful handling and storage.
-
Americium (Am): Found in smoke detectors as an alpha particle emitter, americium is a testament to the versatility of actinides despite their inherent radioactivity.
Periodic Trends in Period 6
Period 6 exhibits clear trends in several key properties. Understanding these trends provides valuable insights into the chemical behavior of the elements:
Atomic Radius
Atomic radius generally increases across a period until the transition metals, then decreases slightly as the effective nuclear charge increases. The lanthanide and actinide contractions are noticeable phenomena where the atomic radii are smaller than expected due to the poor shielding effect of the f-electrons.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This trend is influenced by the increasing nuclear charge and the relatively ineffective shielding provided by the f-electrons in the lanthanides and actinides.
Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period, reflecting the increase in effective nuclear charge. However, the transition metals show less variation in electronegativity compared to the main group elements.
Reactivity
Reactivity varies considerably across Period 6. The alkali metal (Francium, Fr) is highly reactive, readily losing its outermost electron to form a +1 ion. The noble gases (Radon, Rn) are inert, exhibiting minimal reactivity. The reactivity of transition metals is more complex, often exhibiting multiple oxidation states and forming diverse compounds. The lanthanides and actinides show unique reactivity patterns, often dictated by their oxidation states and complex formation.
Applications and Significance of Period 6 Elements
The elements of Period 6 have profound impacts across numerous industries and technologies:
-
Nuclear Power Generation: Uranium is crucial for nuclear power production, offering a low-carbon energy source.
-
Catalysis: Platinum and other transition metals are essential catalysts in various chemical processes, including automobile catalytic converters.
-
High-Strength Alloys: Tungsten and other transition metals contribute to the strength and durability of high-performance alloys used in aerospace and other demanding applications.
-
Electronics: Gold and other elements find use in electronics, contributing to conductivity and reliability.
-
Medical Applications: Certain lanthanides have applications in medical imaging and therapies.
-
Lighting: Lanthanides contribute to the vibrant colors in fluorescent and LED lighting technologies.
-
Magnets: Neodymium magnets are essential components in various technologies, from wind turbines to electric vehicle motors.
Challenges and Future Directions
While Period 6 elements offer significant benefits, challenges remain:
-
Radioactivity: The radioactivity of actinides presents safety concerns requiring rigorous handling and storage protocols.
-
Environmental Impact: Mining and processing of rare earth elements can have environmental impacts, necessitating sustainable practices.
-
Recycling: The efficient recycling of valuable Period 6 elements is crucial for economic and environmental reasons.
-
Exploring new applications: Further research continues to explore the potential of these elements in emerging technologies like quantum computing and advanced materials science.
Conclusion: A Pivotal Period in the Periodic Table
Period 6 occupies a pivotal position in the periodic table. The inclusion of the f-block elements significantly expands the chemical diversity observed. The elements of Period 6 are indispensable to modern technologies and industries, impacting everything from energy production to electronics and medical applications. While challenges remain in terms of sustainability and safety, ongoing research continues to uncover the remarkable potential of these elements, paving the way for even more innovative applications in the future. The comprehensive understanding of the properties and applications of Period 6 elements is essential for scientific advancement and technological progress.
Latest Posts
Latest Posts
-
The Energy Needed To Start A Chemical Reaction Is Called
Mar 31, 2025
-
Person In Environment In Social Work
Mar 31, 2025
-
What Is The Funnel Used For
Mar 31, 2025
-
Chains Of Carbon Atoms Bonded To Hydrogen Atoms
Mar 31, 2025
-
How To Write Compounds In Chemistry
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Period 6 On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
