What 3 Subatomic Particles Make Up An Atom
Muz Play
Mar 26, 2025 · 6 min read
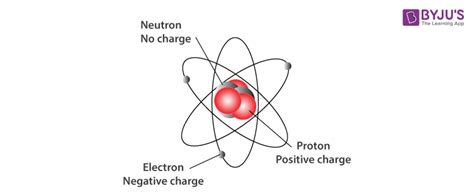
Table of Contents
What 3 Subatomic Particles Make Up an Atom? A Deep Dive into Atomic Structure
The atom, once considered the fundamental building block of matter, is now understood to be a complex system composed of even smaller constituents: subatomic particles. While many subatomic particles exist, three are crucial to understanding the structure and behavior of atoms: protons, neutrons, and electrons. This article will delve deep into the properties, characteristics, and roles of each of these particles, exploring their contributions to atomic structure and the implications for chemistry and physics.
Understanding the Nucleus: The Home of Protons and Neutrons
The heart of an atom is its nucleus, a tiny, dense region at the atom's center. This nucleus houses two of the three key subatomic particles: protons and neutrons. These particles are significantly more massive than electrons and contribute almost entirely to the atom's overall mass.
Protons: The Positively Charged Core
Protons are positively charged particles with a charge of +1 elementary charge. Crucially, the number of protons in an atom's nucleus determines its atomic number and thus, its identity as a specific chemical element. For example, hydrogen (H) has one proton, helium (He) has two, and uranium (U) has 92. This number is fundamental because it dictates the element's chemical properties and how it will interact with other elements. The proton's positive charge is essential for the atom's stability, as it attracts the negatively charged electrons.
Key characteristics of protons:
- Charge: +1 elementary charge
- Mass: Approximately 1.6726 × 10^-27 kg (roughly 1836 times the mass of an electron)
- Location: Nucleus
- Role: Defines the element's atomic number and chemical identity.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, carry no net electric charge. They are electrically neutral particles residing within the atom's nucleus alongside protons. While they don't contribute to the atom's charge, neutrons play a vital role in nuclear stability. The number of neutrons in an atom's nucleus can vary even for atoms of the same element, leading to isotopes. Isotopes are atoms with the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, undergoing decay to become more stable.
Key characteristics of neutrons:
- Charge: 0 (neutral)
- Mass: Approximately 1.6749 × 10^-27 kg (slightly more massive than a proton)
- Location: Nucleus
- Role: Contributes to the atom's mass and nuclear stability; isotopes arise from variations in neutron number.
The Electron Cloud: Orbiting Electrons and Chemical Behavior
Unlike protons and neutrons confined within the nucleus, electrons occupy the space surrounding the nucleus in what's often described as an electron cloud. This cloud isn't a uniformly distributed haze; instead, electrons exist in specific regions of probability called orbitals, which are described by quantum mechanics. The electron cloud's size determines the atom's overall size.
Electrons: The Negatively Charged Orbiters
Electrons are negatively charged particles with a charge of -1 elementary charge. Their number typically equals the number of protons in a neutral atom, balancing the positive charge of the protons and making the atom electrically neutral overall. However, atoms can gain or lose electrons, becoming ions (charged atoms). This ability to gain or lose electrons is crucial for chemical bonding and the formation of molecules and compounds.
Key characteristics of electrons:
- Charge: -1 elementary charge
- Mass: Approximately 9.1094 × 10^-31 kg (significantly less massive than protons and neutrons)
- Location: Electron cloud surrounding the nucleus
- Role: Involved in chemical bonding, determining an atom's reactivity and chemical properties.
The Quantum Mechanical Model: Beyond Simple Orbits
The earlier model of electrons orbiting the nucleus like planets around the sun is a simplification. The true behavior of electrons is governed by the principles of quantum mechanics. Electrons occupy orbitals, which are regions of space where the probability of finding an electron is high. These orbitals are characterized by energy levels and shapes (s, p, d, f orbitals), and electrons fill these orbitals according to specific rules. This quantum mechanical description is essential for understanding the chemical behavior of atoms and the formation of chemical bonds.
Isotopes and Their Significance
As mentioned earlier, isotopes are atoms of the same element with the same number of protons but a differing number of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. Many elements have multiple stable isotopes, while others have only radioactive isotopes.
Radioactive Isotopes and Their Applications
Radioactive isotopes are unstable and undergo radioactive decay, emitting particles or energy to become more stable. This decay process is utilized in various applications, including:
- Medical imaging and treatment: Radioactive tracers are used to diagnose and treat diseases.
- Carbon dating: Radioactive carbon-14 is used to determine the age of ancient artifacts.
- Industrial applications: Radioactive isotopes are used in gauging the thickness of materials and in sterilization processes.
The Importance of Subatomic Particles in Chemistry and Physics
The study of subatomic particles is fundamental to both chemistry and physics. In chemistry, understanding the arrangement of electrons determines how atoms interact to form molecules and compounds, explaining chemical reactions and properties. In physics, the study of subatomic particles delves into the fundamental forces of nature, exploring the building blocks of matter and energy at the most basic level. This exploration has led to advancements in various fields, including nuclear energy, materials science, and medical technology.
Beyond Protons, Neutrons, and Electrons: A Glimpse into the Subatomic World
While protons, neutrons, and electrons are the essential particles for understanding atomic structure and basic chemical behavior, it's crucial to acknowledge that the subatomic world is far more complex. Protons and neutrons themselves are composed of even smaller particles called quarks, held together by the strong force. Numerous other subatomic particles exist, including leptons (like electrons and neutrinos) and bosons (force-carrying particles like photons). The study of these particles continues to be a significant area of research in particle physics, providing deeper insights into the fundamental forces and the universe's origins.
Conclusion: The Building Blocks of Matter
The three subatomic particles – protons, neutrons, and electrons – form the fundamental building blocks of atoms, determining their properties and behavior. Understanding their characteristics, interactions, and roles is essential for grasping the intricacies of chemistry, physics, and numerous related scientific fields. While the simple model of protons, neutrons, and electrons provides a solid foundation, the ongoing exploration of subatomic particles reveals a fascinating and complex universe at the heart of matter itself. The journey into the subatomic realm is a continuing adventure, constantly expanding our understanding of the universe and the fundamental forces that govern it. Further research promises to unveil even more profound insights into the very fabric of reality.
Latest Posts
Latest Posts
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
-
What Are The Vertical Columns Called On A Periodic Table
Mar 29, 2025
-
Where Does Fermentation Take Place In A Cell
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What 3 Subatomic Particles Make Up An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
