What Are 3 Subatomic Particles And Their Charges
Muz Play
Mar 29, 2025 · 7 min read
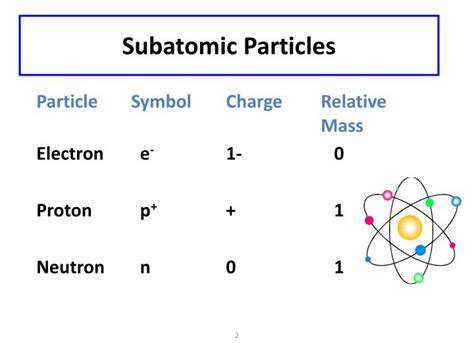
Table of Contents
Delving into the Subatomic World: Exploring Protons, Neutrons, and Electrons
The world around us, from the smallest grain of sand to the largest galaxy, is made up of matter. But what constitutes matter at its most fundamental level? The answer lies in the realm of subatomic particles, the tiny building blocks that make up atoms. While there are many subatomic particles, three stand out as the primary components of all atoms: protons, neutrons, and electrons. Understanding their properties, particularly their charges, is crucial to comprehending the structure and behavior of matter.
Understanding Atomic Structure: A Foundation for Subatomic Particles
Before diving into the specifics of protons, neutrons, and electrons, let's briefly review the basic atomic structure. An atom is the smallest unit of an element that retains the chemical properties of that element. At the atom's center lies the nucleus, a dense region containing protons and neutrons. Surrounding the nucleus is a cloud of electrons orbiting at various energy levels. This arrangement is often depicted as a miniature solar system, with the nucleus as the sun and electrons as planets. However, this model is a simplification; the actual behavior of electrons is far more complex and governed by quantum mechanics.
1. Protons: The Positively Charged Core
Protons are subatomic particles found within the atom's nucleus. Their defining characteristic is their positive electric charge, which is equal in magnitude but opposite in sign to the charge of an electron. This positive charge is a fundamental property; it's not something that can be removed or altered. The number of protons in an atom's nucleus defines the element's atomic number and determines its position on the periodic table. For example, hydrogen has one proton, helium has two, and so on.
Mass and Significance of Protons
Protons possess a relatively large mass compared to electrons, approximately 1,836 times greater. This mass contributes significantly to the overall mass of the atom. The strong nuclear force, one of the four fundamental forces of nature, binds protons together within the nucleus, overcoming the electrostatic repulsion between their positive charges. Without this strong force, the nucleus would simply fly apart.
Isotopes and Proton Number
While the number of protons determines the element, the number of neutrons can vary, leading to the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. These isotopes exhibit similar chemical properties but may differ in their physical properties like mass and radioactive behavior.
2. Neutrons: The Neutral Nucleus Inhabitants
Neutrons, as their name suggests, are electrically neutral subatomic particles. They carry no net electric charge, a key distinction from protons and electrons. Like protons, neutrons reside within the atom's nucleus and are bound together by the strong nuclear force. The number of neutrons in an atom's nucleus, along with the number of protons, determines the atom's mass number (total number of nucleons).
Neutrons' Role in Nuclear Stability
Neutrons play a crucial role in nuclear stability. The presence of neutrons helps to balance the repulsive forces between positively charged protons, preventing the nucleus from disintegrating. The optimal neutron-to-proton ratio varies depending on the element, and deviations from this ratio can lead to unstable nuclei that undergo radioactive decay.
Neutron Discovery and Significance
The discovery of the neutron in 1932 by James Chadwick revolutionized our understanding of atomic structure. Before its discovery, the model of the atom was incomplete. The neutron's role in nuclear stability and its involvement in nuclear reactions (including fission and fusion) are paramount in various scientific fields, including nuclear physics and energy production.
3. Electrons: The Negatively Charged Orbiters
Electrons are subatomic particles that orbit the atom's nucleus at various energy levels. Unlike protons and neutrons, electrons are much lighter, possessing a mass significantly smaller than that of a proton or neutron. Their most defining characteristic is their negative electric charge, equal in magnitude to the positive charge of a proton.
Electron Shells and Energy Levels
Electrons are arranged in shells or energy levels surrounding the nucleus. Electrons in the inner shells are closer to the nucleus and have lower energy than electrons in outer shells. The arrangement of electrons in these shells determines the atom's chemical properties and its ability to form chemical bonds with other atoms.
Electron Behavior and Quantum Mechanics
The behavior of electrons cannot be described by classical mechanics. Instead, their behavior is governed by the principles of quantum mechanics, a branch of physics dealing with the very small. Electrons exhibit wave-particle duality, meaning they exhibit properties of both waves and particles. Their exact position and momentum cannot be precisely determined simultaneously, as stated by the Heisenberg Uncertainty Principle.
Ions and Electron Transfer
The number of electrons in an atom can change, leading to the formation of ions. If an atom loses one or more electrons, it becomes a positive ion (cation), as it has more protons than electrons. Conversely, if an atom gains one or more electrons, it becomes a negative ion (anion), possessing more electrons than protons. This electron transfer is crucial in chemical reactions and the formation of ionic compounds.
The Strong Nuclear Force: The Glue that Holds the Nucleus Together
The strong nuclear force is one of the four fundamental forces of nature and is responsible for binding protons and neutrons together within the atomic nucleus. This force is incredibly strong at very short distances, much stronger than the electromagnetic force that repels positively charged protons. However, its influence diminishes rapidly with distance.
The Electromagnetic Force: Governing Interactions Between Charged Particles
The electromagnetic force is another fundamental force that plays a crucial role in the interactions between charged particles. It governs the attraction between oppositely charged particles (like protons and electrons) and the repulsion between similarly charged particles (like two protons). This force is responsible for the structure of atoms and the formation of molecules through chemical bonds.
Subatomic Particles Beyond the Basics: A Glimpse into a Larger World
While protons, neutrons, and electrons are the primary subatomic particles forming the atoms that make up our everyday world, they are not the only ones. The Standard Model of particle physics describes a vast array of other subatomic particles, including quarks, leptons, and bosons.
Quarks: The Constituents of Protons and Neutrons
Protons and neutrons are not fundamental particles; they are made up of smaller particles called quarks. Each proton and neutron consists of three quarks bound together by the strong nuclear force. There are six types (or "flavors") of quarks: up, down, charm, strange, top, and bottom. Protons are composed of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks.
Leptons: Including the Familiar Electron
Electrons belong to a group of fundamental particles called leptons. Leptons are elementary particles that do not experience the strong nuclear force. Besides electrons, other leptons include muons and tau particles, along with their corresponding neutrinos.
Bosons: Force Carriers
Bosons are force-carrying particles that mediate the interactions between other particles. For example, photons are bosons that mediate the electromagnetic force, while gluons mediate the strong nuclear force. The exchange of these bosons explains how forces act between particles.
Conclusion: A Journey into the Infinitesimal
The exploration of subatomic particles and their charges is a fascinating journey into the fundamental building blocks of matter. Understanding the properties of protons, neutrons, and electrons—their masses, charges, and interactions—is crucial for comprehending the behavior of atoms, molecules, and ultimately, all matter in the universe. While the Standard Model provides a robust framework for understanding subatomic particles, ongoing research continues to refine our knowledge and uncover new discoveries in this exciting field of physics. The seemingly simple structure of the atom hides a complex and dynamic world waiting to be explored further.
Latest Posts
Latest Posts
-
Four Ways To Represent A Function
Mar 31, 2025
-
What Is The Functional Unit Of Heredity
Mar 31, 2025
-
Cracking The Code Of Life Answer Key
Mar 31, 2025
-
Delta H Is Negative Exothermic Or Endothermic
Mar 31, 2025
-
Raising And Lowering Operators Angular Momentum
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are 3 Subatomic Particles And Their Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
