What Are Families On The Periodic Table
Muz Play
Mar 29, 2025 · 6 min read
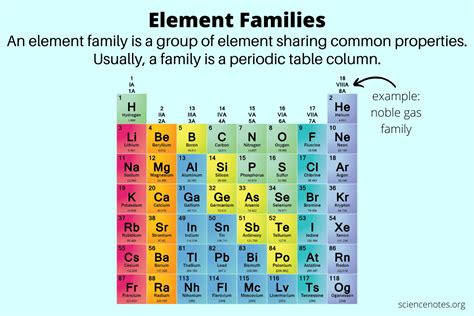
Table of Contents
What Are Families on the Periodic Table? Understanding Group Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. While periods (rows) represent increasing electron shells, families or groups (columns) are characterized by elements sharing similar chemical behaviors. This similarity stems from the identical number of valence electrons – the electrons in the outermost shell that participate in chemical bonding. Understanding these families is crucial for predicting the properties and reactivity of elements.
The Noble Gases: The Unreactive Family
Located in Group 18 (VIIIA), the noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) are famously inert. Their valence shells are completely filled, making them exceptionally stable and reluctant to participate in chemical reactions. This lack of reactivity is why they were once called "inert gases." However, under extreme conditions, some heavier noble gases can form compounds.
Key Characteristics of Noble Gases:
- Full valence electron shells: This leads to exceptional stability and low reactivity.
- Monatomic gases: They exist as single atoms, not molecules.
- Colorless, odorless, and tasteless: Their lack of reactivity also contributes to their lack of noticeable properties.
- Low boiling points: Reflecting their weak interatomic forces.
- Limited applications: Primarily used in lighting (neon signs), welding (argon), and medical imaging (xenon).
The Alkali Metals: Highly Reactive Group 1
Group 1, the alkali metals (Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium), are highly reactive metals. Each possesses a single valence electron, readily lost to achieve a stable electron configuration. This ease of electron donation makes them highly reactive with water and other substances.
Key Characteristics of Alkali Metals:
- One valence electron: Easily lost, resulting in a +1 ion.
- Low ionization energies: Relatively easy to remove the valence electron.
- Soft and silvery-white: Their metallic character is evident in their physical appearance.
- Low densities: Some are even less dense than water (e.g., Lithium).
- Reactive with water: Produce hydrogen gas and a metal hydroxide, often with a vigorous reaction.
- Applications: Used in batteries (lithium-ion batteries), streetlights (sodium lamps), and various industrial processes.
The Alkaline Earth Metals: Reactive Group 2
Group 2, the alkaline earth metals (Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium), are also reactive metals, though less so than the alkali metals. They possess two valence electrons, readily lost to form +2 ions. Their reactivity increases down the group.
Key Characteristics of Alkaline Earth Metals:
- Two valence electrons: Easily lost to form a +2 ion.
- Higher ionization energies than alkali metals: More energy is required to remove the two valence electrons.
- Harder and denser than alkali metals: Reflecting stronger metallic bonding.
- Reactive with water (except Beryllium): The reaction becomes more vigorous down the group.
- Applications: Magnesium in alloys (lightweight materials), Calcium in bones and teeth, and Beryllium in specialized applications requiring high strength and rigidity.
The Halogens: Highly Reactive Nonmetals in Group 17
Group 17, the halogens (Fluorine, Chlorine, Bromine, Iodine, and Astatine), are highly reactive nonmetals. They have seven valence electrons, readily gaining one electron to achieve a stable octet. This high reactivity makes them important in many chemical processes.
Key Characteristics of Halogens:
- Seven valence electrons: Readily gain one electron to form a -1 ion.
- High electronegativities: Strongly attract electrons in chemical bonds.
- Varying states at room temperature: Fluorine and Chlorine are gases, Bromine is a liquid, and Iodine is a solid.
- Reactive with metals: Form ionic compounds (salts).
- Applications: Fluorine in dental hygiene, Chlorine in water purification, and Iodine in medical applications.
The Transition Metals: A Diverse Family in the Middle
The transition metals occupy the central block of the periodic table. They are characterized by partially filled d orbitals, leading to variable oxidation states and a wide range of chemical properties. Their properties are less predictable based solely on their group number than those of the main group elements.
Key Characteristics of Transition Metals:
- Partially filled d orbitals: Allows for variable oxidation states.
- Formation of colored compounds: Due to electronic transitions within the d orbitals.
- Catalytic activity: Many transition metals act as catalysts in chemical reactions.
- High melting and boiling points: Strong metallic bonding contributes to their high thermal stability.
- Formation of complex ions: Transition metals readily form complex ions with ligands.
- Applications: Wide-ranging applications, including construction (iron, steel), electronics (copper, gold), and catalysis (platinum, palladium).
The Lanthanides and Actinides: The Inner Transition Metals
The lanthanides (rare earth elements) and actinides are placed separately at the bottom of the periodic table. They are characterized by filling of the 4f and 5f orbitals, respectively. They share similar chemical properties within their respective series.
Key Characteristics of Lanthanides and Actinides:
- Filling of f orbitals: Leads to similar chemical properties within each series.
- Similar chemical properties within each series: Making separation and purification challenging.
- Many are radioactive: Particularly the actinides.
- Applications: Wide-ranging applications, including magnets (lanthanides), nuclear fuel (actinides), and various specialized applications.
Understanding Trends within Families
As you move down a group on the periodic table, several trends emerge:
- Atomic radius increases: More electron shells are added.
- Ionization energy generally decreases: Electrons are farther from the nucleus and less tightly bound.
- Electronegativity generally decreases: Atoms become less likely to attract electrons.
- Reactivity generally increases (for metals) or decreases (for nonmetals): This is due to changes in atomic size and electron shielding.
These trends are crucial for understanding the reactivity and chemical behavior of elements within a particular family. For example, the increasing reactivity of alkali metals down the group explains why Cesium reacts more violently with water than Lithium. Similarly, the decreasing reactivity of halogens explains why Fluorine is a far more powerful oxidizing agent than Iodine.
Beyond the Basic Families: More nuanced groupings
While the major families discussed above provide a fundamental understanding of periodic table organization, more nuanced groupings exist within the broader framework. For example, within the transition metals, there are subgroups exhibiting unique characteristics. Some transition metals are known for their magnetic properties, others for their catalytic abilities, and still others for their ability to form coordination complexes. Similarly, within the main group elements, the subtle differences in reactivity and properties lead to further categorization within those families.
The Importance of Understanding Families
Understanding the families of the periodic table is essential for a variety of reasons. It allows chemists to:
- Predict the properties of elements: Knowing the family of an element allows for a general prediction of its physical and chemical properties.
- Design new materials: Understanding the properties of different elements allows for the design of new materials with specific characteristics.
- Understand chemical reactions: Knowing how elements in a particular family react allows for a better understanding of chemical reactions.
- Develop new technologies: Many technologies rely on the unique properties of specific elements and their families.
In conclusion, the families of the periodic table are crucial for understanding the organization and properties of chemical elements. Their systematic arrangement allows for predictions of reactivity, facilitates the design of new materials, and provides a fundamental framework for advancing chemical knowledge and innovation. The exploration of each family reveals a fascinating interplay of atomic structure, electron configuration, and chemical behavior, highlighting the power and elegance of the periodic table as a predictive tool in chemistry. Continued research and exploration of these families will continue to unlock new possibilities in various scientific fields.
Latest Posts
Latest Posts
-
What Is The Difference Between Density Dependent And Density Independent
Mar 31, 2025
-
10 Common Diseases That Can Cause A Secondary Immunodeficiency
Mar 31, 2025
-
Factoring Trinomials With A Leading Coefficient
Mar 31, 2025
-
What Is The Coefficient Of Restitution
Mar 31, 2025
-
What Are The Basic Units Of All Living Things
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are Families On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
