What Are Group Two Elements Called
Muz Play
Mar 25, 2025 · 6 min read
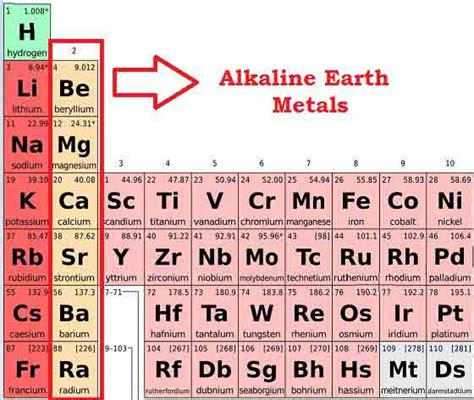
Table of Contents
What Are Group 2 Elements Called? Exploring the Alkaline Earth Metals
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the arrangement and characteristics of these elements is crucial to comprehending the world around us. This article delves into Group 2 elements, commonly known as alkaline earth metals. We'll explore their properties, reactivity, applications, and the unique characteristics that set them apart.
Defining the Alkaline Earth Metals: Group 2 Elements
Group 2, also known as IIA in older numbering systems, comprises six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements are collectively termed alkaline earth metals due to their shared properties. The name "alkaline earth" originates from their oxides, which are alkaline (basic) and were historically found in earthy minerals.
Key Characteristics of Alkaline Earth Metals
The alkaline earth metals share several distinguishing features:
-
Two Valence Electrons: Each alkaline earth metal possesses two electrons in its outermost shell (valence shell). This configuration dictates their chemical behavior, leading to a +2 oxidation state. This means they readily lose two electrons to achieve a stable electron configuration, similar to the noble gases.
-
Metallic Properties: All alkaline earth metals are metals, exhibiting typical metallic properties like good electrical and thermal conductivity, malleability (ability to be hammered into sheets), and ductility (ability to be drawn into wires). However, their metallic character varies across the group, with beryllium being the least metallic and radium the most.
-
Reactivity: While less reactive than the alkali metals (Group 1), alkaline earth metals are still reactive, particularly with water and oxygen. Their reactivity increases as you move down the group. Beryllium is relatively unreactive, while calcium, strontium, barium, and radium react vigorously with water.
-
Formation of Ionic Compounds: Due to their tendency to lose two electrons, alkaline earth metals primarily form ionic compounds, where they exist as +2 cations. These cations readily interact with anions (negatively charged ions) to form stable crystalline structures.
-
Increasing Atomic Radius and Reactivity: As you descend Group 2, the atomic radius increases. This increase leads to a decrease in ionization energy, making it easier for the outer electrons to be removed, resulting in increased reactivity down the group.
Individual Alkaline Earth Metals: A Closer Look
Let's examine each alkaline earth metal in more detail, highlighting their unique characteristics and applications.
1. Beryllium (Be)
Beryllium, the lightest alkaline earth metal, is a strong, lightweight, and brittle metal. Its unique combination of properties makes it valuable in various applications, including:
-
Aerospace Industry: Beryllium's high strength-to-weight ratio makes it ideal for aerospace components, like aircraft and spacecraft parts.
-
Nuclear Reactors: Its low neutron absorption cross-section is crucial in nuclear reactor construction.
-
X-ray windows: Its ability to transmit X-rays makes it suitable for X-ray equipment windows.
-
Electronics: It's used in some specialized electronic components due to its high thermal conductivity.
However, beryllium is toxic, requiring careful handling and safety precautions.
2. Magnesium (Mg)
Magnesium is a relatively abundant alkaline earth metal, widely used in various applications due to its lightness and strength:
-
Alloys: Magnesium alloys are employed in lightweight automotive parts, aircraft components, and sporting goods.
-
Photography: Magnesium is used in flash photography due to its ability to burn brightly.
-
Medicine: Magnesium compounds have essential biological roles and are used in various medicinal applications.
-
Construction: Magnesium is used in some construction materials due to its lightweight and relatively high strength.
3. Calcium (Ca)
Calcium is an abundant element, crucial for biological processes:
-
Bones and Teeth: Calcium is a vital component of bones and teeth, providing structural strength.
-
Muscle Contraction: It plays a critical role in muscle contraction and nerve impulse transmission.
-
Cement and Plaster: Calcium compounds like calcium carbonate (limestone) and calcium sulfate (gypsum) are used in cement and plaster.
-
Metallurgy: Calcium is sometimes used as a reducing agent in metallurgy to produce other metals.
4. Strontium (Sr)
Strontium's applications are less widespread than those of magnesium or calcium. Notable uses include:
-
Pyrotechnics: Strontium salts produce a brilliant red color in fireworks.
-
Nuclear Medicine: Strontium-89 is used in the treatment of bone cancer.
-
Certain Alloys: Strontium is used in small quantities in certain alloys to improve their properties.
5. Barium (Ba)
Barium, like strontium, has limited widespread applications:
-
Medical Imaging: Barium sulfate is used as a contrast agent in medical imaging (X-rays and CT scans).
-
Glass Manufacturing: Barium compounds improve the refractive index of glass.
-
Ceramics: Barium compounds are used in some types of ceramics.
6. Radium (Ra)
Radium is a radioactive element, and its use is extremely limited due to its radioactivity and toxicity:
-
Historically Used in Radiation Therapy: Historically, radium was used in radiation therapy, but safer alternatives are now available.
-
Research Purposes: Radium is primarily used for scientific research purposes.
The high radioactivity of radium necessitates extreme caution in its handling and disposal.
Reactivity of Alkaline Earth Metals and Their Compounds
The reactivity of alkaline earth metals increases down the group. This is because the atomic radius increases, leading to a decrease in ionization energy. The outer electrons are more easily lost, resulting in increased reactivity with water, oxygen, and acids.
-
Reaction with Water: Beryllium doesn't react with water at room temperature. Magnesium reacts slowly with hot water. Calcium, strontium, and barium react vigorously with water, producing hydrogen gas and metal hydroxides.
-
Reaction with Oxygen: All alkaline earth metals react with oxygen to form oxides. The reactivity increases down the group.
-
Reaction with Acids: Alkaline earth metals react with acids to produce hydrogen gas and metal salts.
Applications of Alkaline Earth Metals and Their Compounds
Alkaline earth metals and their compounds have a wide range of applications in various industries:
-
Construction: Calcium compounds like limestone and gypsum are used extensively in construction materials, such as cement and plaster.
-
Metallurgy: Magnesium is used in alloys to reduce weight and improve strength. Calcium is used as a reducing agent in the production of certain metals.
-
Medicine: Magnesium and calcium play crucial roles in biological processes, with magnesium being an essential cofactor in many enzymes and calcium being vital for bone health.
-
Pyrotechnics: Strontium salts produce a vibrant red color in fireworks. Magnesium is also used in some pyrotechnic applications.
-
Nuclear Industry: Beryllium's low neutron absorption cross-section makes it suitable for use in nuclear reactors.
-
Electronics: Beryllium is used in some specialized electronic applications due to its high thermal conductivity.
-
Medical Imaging: Barium sulfate is used as a contrast agent in medical imaging procedures.
Environmental Considerations of Alkaline Earth Metals
While alkaline earth metals have numerous applications, it's crucial to consider their environmental impact. Improper handling and disposal of these metals and their compounds can lead to environmental pollution. Responsible mining, processing, and disposal practices are essential to mitigate potential harm to the environment.
Conclusion: The Importance of Alkaline Earth Metals
The alkaline earth metals, with their unique properties and varied applications, are vital elements in various aspects of modern life. From the construction industry to medicine and aerospace, these metals and their compounds play essential roles. Understanding their properties, reactivity, and applications is fundamental to appreciating their importance in science, technology, and everyday life. Further research into their properties and applications continues to unlock new possibilities for innovation and progress. The study of Group 2 elements remains a fascinating area within the broader field of chemistry, constantly revealing new insights and contributing to advancements across diverse scientific disciplines.
Latest Posts
Latest Posts
-
Free Radical Polymerization Of 2 Chloro 1 3 Butadiene
Mar 28, 2025
-
Why Is Solid Water Less Dense
Mar 28, 2025
-
What Are Signed Numbers In Math
Mar 28, 2025
-
How To Find The Domain Of A Vector Function
Mar 28, 2025
-
Which Muscle Is Not Part Of The Rotator Cuff
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Are Group Two Elements Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
