Why Is Solid Water Less Dense
Muz Play
Mar 28, 2025 · 5 min read
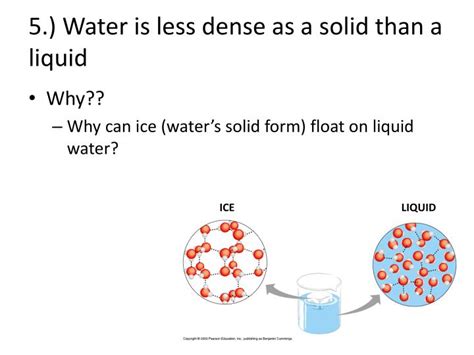
Table of Contents
- Why Is Solid Water Less Dense
- Table of Contents
- Why is Solid Water Less Dense Than Liquid Water? An In-Depth Exploration
- The Hydrogen Bond: The Key Player
- The Crystalline Structure of Ice
- Visualizing the Hexagonal Lattice
- The Role of Hydrogen Bonds in Ice Formation
- Density: A Matter of Packing
- Comparing Liquid Water and Ice
- Implications of Ice's Lower Density
- 1. Aquatic Life
- 2. Global Climate Regulation
- 3. Water Distribution and the Water Cycle
- Exceptions and nuances:
- Conclusion: A Remarkable Property with Profound Consequences
- Latest Posts
- Latest Posts
- Related Post
Why is Solid Water Less Dense Than Liquid Water? An In-Depth Exploration
Water, the elixir of life, is unique in many ways. One of its most remarkable properties, crucial for life on Earth as we know it, is that ice is less dense than liquid water. This seemingly simple fact has profound implications for aquatic ecosystems, weather patterns, and even the very possibility of life thriving on our planet. But why is this the case? Let's delve into the fascinating molecular structure of water to uncover the answer.
The Hydrogen Bond: The Key Player
The unusual behavior of water stems primarily from the hydrogen bond, a special type of dipole-dipole attraction between molecules. Water (H₂O) is a polar molecule, meaning it has a slightly positive end (the hydrogen atoms) and a slightly negative end (the oxygen atom). This polarity allows water molecules to form hydrogen bonds with each other. The slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of a neighboring molecule.
These hydrogen bonds are relatively weak compared to covalent bonds (the bonds within a water molecule), but they are strong enough to significantly influence the properties of water, especially its density. In liquid water, these hydrogen bonds are constantly forming and breaking, creating a dynamic and ever-shifting network.
The Crystalline Structure of Ice
When water freezes, the hydrogen bonds become more organized and stable. The molecules arrange themselves into a crystalline structure, creating a hexagonal lattice with open spaces. This open structure is what accounts for the lower density of ice compared to liquid water.
Visualizing the Hexagonal Lattice
Imagine arranging ping pong balls in a way that maximizes the space between them. You wouldn't stack them directly on top of each other; instead, you'd create a more spacious arrangement. This is analogous to how water molecules arrange themselves in ice. The hexagonal lattice maximizes the hydrogen bonding while leaving considerable void space within the structure.
The Role of Hydrogen Bonds in Ice Formation
The formation of this hexagonal lattice is crucial. As water cools, the kinetic energy of the molecules decreases. This allows the hydrogen bonds to become more stable and ordered. The molecules align themselves to maximize the number of hydrogen bonds, ultimately leading to the formation of the less dense, open crystalline structure.
Density: A Matter of Packing
Density is defined as mass per unit volume. Since the mass of a given amount of water remains relatively constant whether it's liquid or solid, the difference in density lies in the volume occupied. Because the ice crystal structure has these open spaces, it occupies a larger volume than the same mass of liquid water, resulting in a lower density.
Comparing Liquid Water and Ice
In liquid water, the molecules are more tightly packed together, constantly moving and jostling, leading to a denser state. In ice, the regular arrangement of molecules, dictated by the hydrogen bonds, results in a more open, less dense structure. This is why ice floats on water; a less dense substance will always float on a denser substance.
Implications of Ice's Lower Density
The fact that ice is less dense than water has profound consequences for the world around us:
1. Aquatic Life
The fact that ice floats acts as an insulator, preventing bodies of water from freezing solid from the bottom up. This creates a habitable environment for aquatic organisms even during harsh winters. If ice were denser than water, it would sink to the bottom, leading to the complete freezing of lakes and oceans, potentially devastating aquatic ecosystems.
2. Global Climate Regulation
The floating ice layer on the surface of water plays a crucial role in regulating global climate. It reflects sunlight back into space, helping to maintain Earth’s temperature and preventing excessive warming. This insulation effect also influences the rate of sea ice formation and melting, which are significant factors affecting global climate patterns.
3. Water Distribution and the Water Cycle
The lower density of ice also affects water distribution and the water cycle. Glaciers and ice caps, formed from frozen water, store vast quantities of freshwater. The melting of these ice formations affects sea levels and water availability in different parts of the world. Understanding the properties of ice and its density is crucial for modeling and predicting climate change and its impact on global water resources.
Exceptions and nuances:
While the principle of ice being less dense than water is generally true, there are some nuances and exceptions:
- High-pressure ice: Under extreme pressure, various forms of ice exist with different crystal structures and densities. Some of these high-pressure ice forms are actually denser than liquid water. This demonstrates that the hydrogen bonding arrangement is highly sensitive to environmental conditions.
- Supercooled water: Liquid water can be cooled below its freezing point (0°C or 32°F) without freezing, a phenomenon known as supercooling. In this state, the water is metastable, meaning it is unstable and likely to freeze rapidly if disturbed. The density of supercooled water is slightly higher than that of ice but lower than that of regular liquid water at the same temperature.
Conclusion: A Remarkable Property with Profound Consequences
The fact that solid water (ice) is less dense than liquid water is a truly remarkable property. It arises from the unique structure of the water molecule and the nature of hydrogen bonds. This seemingly simple difference in density has profound and far-reaching implications for life on Earth, climate regulation, and the global water cycle. Understanding this fundamental property is essential for appreciating the complexity and wonder of the natural world and for addressing the challenges posed by climate change and resource management. Further research into the intricate behavior of water, particularly under extreme conditions, continues to reveal fascinating insights into this essential substance. From the microscopic dance of molecules to the macroscopic scale of glaciers and oceans, the story of water's density is a testament to the power of simple principles to shape our planet and all life upon it.
Latest Posts
Latest Posts
-
Active Sites On The Actin Become Available For Binding After
Apr 01, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Apr 01, 2025
-
Which Statement About Immigration Federalism Is False
Apr 01, 2025
-
Which Will Increase The Rate Of A Chemical Reaction
Apr 01, 2025
-
How Are Electrons Arranged Around The Nucleus Of An Atom
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Is Solid Water Less Dense . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
