Which Will Increase The Rate Of A Chemical Reaction
Muz Play
Apr 01, 2025 · 6 min read
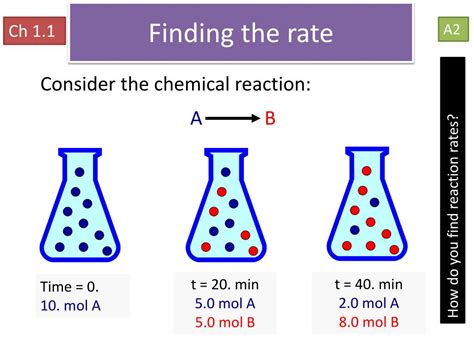
Table of Contents
Factors Affecting the Rate of Chemical Reactions: A Comprehensive Guide
Chemical reactions are the foundation of our world, from the rusting of iron to the processes within our bodies. Understanding what influences the speed of these reactions – the reaction rate – is crucial in many fields, from industrial chemistry to medicine. This comprehensive guide delves into the key factors that significantly impact the rate of a chemical reaction.
1. Nature of Reactants: The Intrinsic Influence
The inherent properties of the reacting substances play a foundational role in determining reaction rate. Some reactions are naturally faster than others, regardless of external conditions. This is primarily due to:
1.1 Bond Strength and Type:
Stronger bonds require more energy to break, thus slowing down the reaction. For example, reactions involving the breaking of triple bonds (like in nitrogen gas, N₂) are generally slower than those involving single bonds. The type of bond also matters – ionic bonds are typically easier to break than covalent bonds, leading to faster reactions for ionic compounds.
1.2 Molecular Structure and Steric Hindrance:
The spatial arrangement of atoms within molecules significantly influences reaction rates. Steric hindrance, where bulky groups around the reactive site impede the approach of other molecules, dramatically slows down reactions. Imagine trying to fit a large key into a small lock – it's difficult! Similarly, large molecules might struggle to collide effectively, reducing reaction frequency.
1.3 Reactivity of Functional Groups:
Different functional groups within molecules exhibit varying reactivities. Certain groups, like highly electronegative atoms or those with easily polarized bonds, are inherently more reactive and participate in faster reactions. This understanding is fundamental in organic chemistry, where the reactivity of functional groups dictates the outcome of countless reactions.
2. Concentration of Reactants: More Means Faster
A higher concentration of reactants translates directly to a greater likelihood of collisions between reacting molecules. This increased collision frequency leads to a faster reaction rate. This is because, for a reaction to occur, reactant molecules must first collide with sufficient energy (activation energy) and the correct orientation. More molecules mean more chances for successful collisions.
This principle is mathematically expressed in the rate law, which relates reaction rate to reactant concentrations. For a simple reaction, A + B → C, the rate law might be expressed as:
Rate = k[A][B]
where:
- 'k' is the rate constant (specific to the reaction)
- [A] and [B] represent the concentrations of reactants A and B.
This equation demonstrates the direct proportionality between reactant concentration and reaction rate.
3. Temperature: The Energy Booster
Temperature plays a pivotal role by affecting the kinetic energy of reactant molecules. Higher temperatures translate to higher kinetic energies, resulting in more frequent and more energetic collisions. Crucially, a higher proportion of these collisions will possess the necessary activation energy – the minimum energy required for a reaction to occur. This means a greater number of successful collisions, leading to a significant increase in reaction rate.
The Arrhenius equation quantifies this relationship:
k = Ae^(-Ea/RT)
where:
- 'k' is the rate constant
- 'A' is the pre-exponential factor (frequency of collisions)
- 'Ea' is the activation energy
- 'R' is the ideal gas constant
- 'T' is the temperature in Kelvin
This equation illustrates the exponential dependence of the rate constant (and therefore the reaction rate) on temperature. A small increase in temperature can cause a substantial increase in reaction rate.
4. Surface Area: More Contact, Faster Reaction
For reactions involving solids, the surface area exposed to the reactants is critical. A larger surface area provides more contact points for collisions, thus increasing the reaction rate. Consider the combustion of wood: a finely powdered wood dust will burn much more rapidly than a large log because the dust offers a drastically larger surface area. This principle is exploited in many industrial processes, where catalysts are often finely divided to maximize their effectiveness.
5. Catalysts: The Reaction Accelerators
Catalysts are substances that increase the rate of a reaction without being consumed themselves. They achieve this by providing an alternative reaction pathway with a lower activation energy. By lowering the energy barrier, a larger fraction of collisions possess the required energy to overcome it, thus speeding up the reaction. Enzymes, biological catalysts, are a prime example of this principle, enabling the rapid and specific biochemical reactions necessary for life.
Catalysts work through various mechanisms, including:
- Providing an alternative reaction pathway: The catalyst forms an intermediate complex with one or more reactants, lowering the activation energy.
- Orienting reactants: The catalyst can hold reactant molecules in a favorable orientation, increasing the likelihood of successful collisions.
6. Pressure: Compressing for Faster Reactions
For reactions involving gases, increasing the pressure increases the concentration of the gaseous reactants. As explained earlier, higher concentrations lead to a higher rate of successful collisions, thus boosting the reaction rate. This is especially significant for reactions where the number of gaseous molecules changes during the reaction.
7. Light: Photochemical Reactions
Some reactions, known as photochemical reactions, require light to proceed. Light provides the energy needed to initiate the reaction, often by exciting molecules to a higher energy state, making them more reactive. Photosynthesis, the process by which plants convert light energy into chemical energy, is a classic example of a photochemical reaction.
8. Presence of Inhibitors: Slowing Things Down
In contrast to catalysts, inhibitors are substances that decrease the rate of a reaction. They can achieve this by various mechanisms, such as:
- Blocking active sites: In reactions involving catalysts, inhibitors can occupy the active sites of the catalyst, preventing reactant molecules from binding and reacting.
- Reacting with intermediates: Inhibitors might react with intermediate species formed during the reaction, disrupting the reaction pathway.
Optimizing Reaction Rates: Practical Applications
Understanding these factors allows us to manipulate reaction rates for various purposes. In industrial settings, optimizing reaction rates is crucial for efficiency and cost-effectiveness. For example, controlling temperature, pressure, and catalyst choice can significantly impact the yield and speed of a chemical process.
In the pharmaceutical industry, understanding reaction rates is essential for designing efficient drug synthesis routes and optimizing drug delivery systems. In environmental science, understanding reaction rates helps in predicting the fate of pollutants and designing effective remediation strategies.
Conclusion: A Multifaceted Influence
The rate of a chemical reaction is a complex interplay of various factors. While the nature of the reactants sets the stage, external conditions like temperature, concentration, pressure, surface area, the presence of catalysts or inhibitors, and even light can dramatically influence the speed of a reaction. By understanding these factors, we can predict, control, and optimize reaction rates across diverse scientific and technological fields. This knowledge forms the backbone of chemical engineering, pharmaceutical development, environmental remediation, and numerous other areas where controlling the speed of chemical transformations is paramount.
Latest Posts
Latest Posts
-
How Are Ionic And Covalent Bonding Similar
Apr 02, 2025
-
What Is The Electron Configuration Of Neon
Apr 02, 2025
-
In Which Phase Of Cellular Respiration Is Water Made
Apr 02, 2025
-
How To Measure Current On A Breadboard
Apr 02, 2025
-
Chapter 1 Lab Investigation The Language Of Anatomy
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Will Increase The Rate Of A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
