What Are The Factors Affecting The Rate Of Diffusion
Muz Play
Apr 01, 2025 · 7 min read
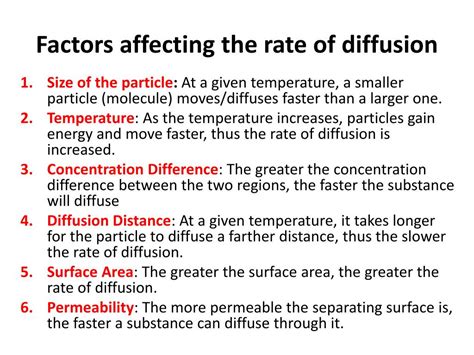
Table of Contents
What are the Factors Affecting the Rate of Diffusion?
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in many biological and physical systems. Understanding the factors that influence the rate of diffusion is crucial in various fields, from medicine and pharmacology to environmental science and materials engineering. This article delves into the key factors governing diffusion rates, explaining their mechanisms and providing illustrative examples.
1. Concentration Gradient
The concentration gradient, the difference in concentration between two regions, is arguably the most significant factor affecting the rate of diffusion. A steeper concentration gradient results in a faster rate of diffusion. This is because the higher the difference in concentration, the greater the driving force pushing particles from the high-concentration area to the low-concentration area. Think of it like a crowded room – people will disperse more quickly if there's a much larger, emptier space next door compared to a slightly less crowded room.
The mathematical representation of this relationship is often described by Fick's First Law of Diffusion, which states that the flux (J) of a substance is proportional to the negative concentration gradient:
J = -D (dC/dx)
Where:
- J is the diffusion flux (amount of substance crossing a unit area per unit time)
- D is the diffusion coefficient (a measure of how easily a substance diffuses through a medium)
- dC/dx is the concentration gradient (change in concentration over distance)
Examples of Concentration Gradient's Influence
- Oxygen diffusion in the lungs: The steep concentration gradient between the alveoli (high oxygen concentration) and the capillaries (low oxygen concentration) facilitates rapid oxygen uptake into the bloodstream.
- Nutrient uptake in plant roots: The higher concentration of nutrients in the soil compared to the root cells drives efficient nutrient absorption.
- Perfume scent dispersal: The high concentration of perfume molecules in the bottle leads to their rapid diffusion into the surrounding air.
2. Temperature
Temperature significantly impacts the rate of diffusion. Higher temperatures lead to faster diffusion rates. This is because increased temperature provides particles with greater kinetic energy. With more kinetic energy, particles move faster and collide more frequently, resulting in a more rapid net movement from high to low concentration areas.
The Kinetic Energy Connection
The increased kinetic energy at higher temperatures overcomes intermolecular forces, allowing particles to more easily navigate through the medium. This is especially true for liquids and gases where intermolecular forces are weaker than in solids.
Examples of Temperature's Effect
- Sugar dissolving in hot water: Sugar dissolves faster in hot water because the higher temperature increases the kinetic energy of both sugar and water molecules, facilitating faster diffusion and dissolution.
- Gas diffusion in the atmosphere: Gases diffuse more rapidly in warm air due to the increased kinetic energy of gas molecules.
- Enzyme activity: Enzyme-catalyzed reactions are temperature-dependent. Increased temperature initially speeds up the reaction due to increased diffusion of reactants to the enzyme active site, but excessively high temperatures can denature the enzyme, slowing the reaction down.
3. Surface Area
The surface area available for diffusion directly affects the rate. A larger surface area allows for more particles to simultaneously cross the boundary between regions of different concentrations, resulting in faster diffusion. The more points of contact between the regions, the quicker the diffusion process.
Examples of Surface Area's Impact
- Lungs' alveoli: The lungs' enormous surface area, achieved by the numerous alveoli, maximizes oxygen uptake from the inhaled air.
- Small intestine villi: The villi in the small intestine increase the surface area for efficient nutrient absorption.
- Powdered substances dissolving: Powdered substances dissolve faster than solid chunks because the powdered form offers a much larger surface area for interaction with the solvent.
4. Medium of Diffusion
The medium through which diffusion occurs significantly influences the rate. Diffusion rates are generally faster in gases than in liquids, and faster in liquids than in solids. This difference stems from the varying degrees of freedom particles have in different states of matter. Gases have the most freedom of movement, followed by liquids, and then solids.
Additionally, the viscosity and density of the medium play a role. A less viscous and less dense medium allows for faster diffusion.
Examples: Medium's Influence
- Oxygen diffusion in air vs. water: Oxygen diffuses much faster in air than in water due to the lower density and viscosity of air. This is why aquatic animals have specialized adaptations for oxygen uptake.
- Diffusion through cell membranes: The lipid bilayer of cell membranes acts as a selective barrier, affecting the diffusion rates of different molecules. Small, nonpolar molecules diffuse faster than large, polar molecules.
- Diffusion in porous materials: The pore size and structure in porous materials influence the rate of diffusion. Larger pores generally allow for faster diffusion.
5. Distance
The distance over which diffusion must occur is inversely proportional to the rate. The further the distance, the slower the diffusion rate. This is simply because particles have to travel a greater distance to reach the region of lower concentration.
Examples: The Distance Factor
- Nutrient transport in large organisms: Diffusion alone is inefficient for transporting nutrients over long distances in large multicellular organisms; specialized circulatory systems are essential.
- Drug delivery systems: Drug delivery systems are often designed to minimize the distance drugs need to travel to reach their target sites.
- Gas exchange in large insects: Although insects rely on diffusion for gas exchange, their tracheal systems branch extensively to minimize diffusion distances.
6. Molecular Size and Weight
The size and weight of the diffusing particles directly affect their diffusion rate. Smaller and lighter molecules diffuse faster than larger and heavier ones. This is because smaller molecules experience less resistance as they move through the medium.
Examples: Size Matters
- Gas diffusion: Smaller gas molecules like helium diffuse faster than larger ones like xenon.
- Solute diffusion in solution: Smaller solute molecules diffuse more quickly than larger ones.
- Membrane permeability: The size and shape of molecules influence their ability to pass through cell membranes. Small, hydrophobic molecules pass through more easily than large, hydrophilic molecules.
7. Pressure
In the context of gases, pressure influences the diffusion rate. Higher pressure leads to faster diffusion. This is because increased pressure results in a higher concentration of gas molecules, creating a steeper concentration gradient and hence faster diffusion.
Examples: Pressure's Role
- Gas exchange in lungs: The pressure difference between the atmosphere and the alveoli drives oxygen diffusion into the blood.
- Industrial processes: Many industrial processes utilize pressure differences to enhance diffusion rates in chemical reactions or separation processes.
8. Electrical Charge and Electric Field
In the case of charged particles (ions), the presence of an electrical field dramatically affects the diffusion rate. An electric field exerts a force on charged particles, accelerating their movement. The strength and direction of the electric field directly affect the speed and direction of diffusion.
Examples: The Influence of Charge
- Ion transport across cell membranes: Membrane potential and ion channels create electric fields that drive selective ion movement across cell membranes.
- Electrophoresis: Electrophoresis utilizes electric fields to separate charged molecules based on their size and charge, enhancing their diffusion in a controlled manner.
Conclusion
The rate of diffusion is a complex interplay of several factors. While the concentration gradient serves as the primary driving force, temperature, surface area, the medium of diffusion, distance, molecular size and weight, pressure (for gases), and electrical fields (for charged particles) all significantly influence the speed and efficiency of this fundamental process. Understanding these factors is crucial in numerous scientific and engineering applications, from designing efficient drug delivery systems to optimizing industrial processes and comprehending biological phenomena at the cellular and organismal levels. Further research continually refines our understanding of these intricate interactions and their implications for diverse fields.
Latest Posts
Latest Posts
-
How Many Atp Are Produced In Electron Transport Chain
Apr 02, 2025
-
What Does Rolling Without Slipping Mean
Apr 02, 2025
-
The Price Elasticity Of Supply Measures
Apr 02, 2025
-
Does Co Have Dipole Dipole Forces
Apr 02, 2025
-
What Are The Characteristics Of Enzymes
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Factors Affecting The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
