What Are The Properties Of Solids
Muz Play
Apr 06, 2025 · 6 min read
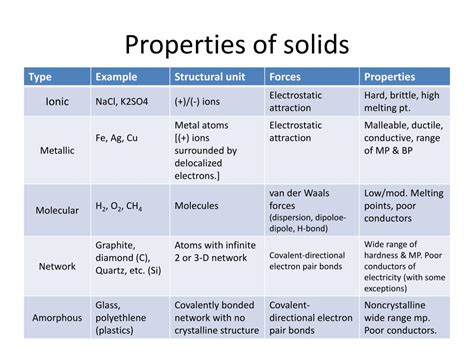
Table of Contents
What are the Properties of Solids? A Deep Dive into the World of Solid Matter
Solids are one of the four fundamental states of matter, distinguished by their rigid structure and definite shape and volume. Understanding their properties is crucial across numerous scientific disciplines, from materials science and engineering to chemistry and physics. This comprehensive guide delves into the diverse characteristics of solids, exploring their defining features, classifications, and the underlying principles that govern their behavior.
Defining Properties of Solids
Solids are characterized by several key properties that differentiate them from liquids and gases. These defining features stem from the strong intermolecular forces holding their constituent particles (atoms, molecules, or ions) together in a fixed arrangement.
1. Definite Shape and Volume:
This is perhaps the most readily observable property of solids. Unlike liquids and gases, which adapt to the shape of their containers, solids retain their shape and volume regardless of their environment. This is due to the strong intermolecular forces that restrict the movement of particles. The particles in a solid are tightly packed and vibrate around fixed positions, preventing significant changes in shape or volume.
2. Incompressibility:
Solids are largely incompressible, meaning their volume doesn't change significantly even under considerable pressure. The close proximity of particles leaves little space for compression. While some degree of compression is possible at extremely high pressures, it's generally negligible compared to liquids and gases.
3. Rigidity and Hardness:
The strong attractive forces between particles in solids give rise to their rigidity and hardness. Rigidity refers to the resistance to deformation, while hardness describes the resistance to scratching or indentation. These properties are influenced by the type of bonding between particles and the arrangement of their crystal lattice. Diamond, for example, possesses exceptional hardness due to its strong covalent bonds and highly ordered structure.
4. High Density:
Compared to liquids and gases, solids generally have higher densities due to the close packing of their constituent particles. The density varies depending on the type of solid and its atomic structure. Dense solids like metals have tightly packed atoms, while less dense solids may have more open structures.
5. Low Rate of Diffusion:
Diffusion, the movement of particles from a region of high concentration to a region of low concentration, occurs at a significantly slower rate in solids than in liquids or gases. This is because the particles in a solid are relatively immobile and their movement is restricted by the strong intermolecular forces.
6. Mechanical Properties:
Solids exhibit a range of mechanical properties including elasticity, plasticity, malleability, and ductility. Elasticity refers to a solid's ability to return to its original shape after the removal of an applied force. Plasticity, on the other hand, is the ability to deform permanently under stress. Malleability is the ability to be hammered or rolled into thin sheets, while ductility refers to the ability to be drawn into wires. These properties are heavily dependent on the type of bonding and crystal structure.
Classification of Solids
Solids can be broadly classified into two main categories based on the arrangement of their constituent particles:
1. Crystalline Solids:
Crystalline solids possess a highly ordered, repeating three-dimensional arrangement of atoms, ions, or molecules. This regular arrangement gives rise to a well-defined crystal lattice, which is responsible for many of the unique properties of crystalline solids. Examples include table salt (NaCl), quartz (SiO2), and diamond (C). The regular arrangement leads to anisotropy, meaning that properties may vary depending on the direction.
- Types of Crystalline Solids: Crystalline solids are further categorized based on the type of bonding between their constituent particles:
- Ionic solids: Held together by electrostatic forces between oppositely charged ions (e.g., NaCl).
- Covalent solids: Held together by covalent bonds between atoms (e.g., diamond).
- Metallic solids: Held together by metallic bonds, where electrons are delocalized across a sea of atoms (e.g., iron, copper).
- Molecular solids: Held together by weaker intermolecular forces such as van der Waals forces or hydrogen bonds (e.g., ice, sugar).
2. Amorphous Solids:
Amorphous solids, also known as non-crystalline solids, lack the long-range order characteristic of crystalline solids. Their atoms, ions, or molecules are arranged randomly, similar to a liquid, but with a fixed shape. Examples include glass, rubber, and many plastics. Amorphous solids are typically isotropic, meaning their properties are the same in all directions.
Further Properties and Considerations
The properties of solids are not solely determined by their crystalline or amorphous nature. Several other factors contribute to their overall characteristics:
1. Melting Point and Boiling Point:
Solids have distinct melting and boiling points, which are the temperatures at which they transition to the liquid and gaseous phases, respectively. These points are determined by the strength of the intermolecular forces holding the particles together. Stronger forces lead to higher melting and boiling points.
2. Thermal and Electrical Conductivity:
The thermal and electrical conductivity of solids vary greatly depending on their composition and structure. Metals, for example, are excellent conductors of both heat and electricity due to the delocalized electrons in their metallic bonds. Insulators, on the other hand, have very low conductivity due to the tightly bound electrons. Semiconductors exhibit intermediate conductivity, with properties that can be manipulated by doping.
3. Optical Properties:
Solids exhibit diverse optical properties, such as transparency, translucency, and opacity. These properties are influenced by the interaction of light with the electrons in the solid. Transparent materials allow light to pass through, while opaque materials absorb or reflect light.
4. Magnetic Properties:
Some solids exhibit magnetic properties, such as ferromagnetism (strong attraction to magnets), paramagnetism (weak attraction), or diamagnetism (weak repulsion). These properties arise from the alignment of electron spins within the material.
5. Mechanical Strength and Fracture:
The mechanical strength and fracture behavior of solids are critical in engineering applications. Tensile strength, compressive strength, and shear strength are important parameters that determine a material's ability to withstand different types of stress. Fracture behavior can range from brittle fracture (sudden catastrophic failure) to ductile fracture (gradual deformation before failure).
Applications and Importance
Understanding the properties of solids is fundamental across various fields:
-
Materials Science and Engineering: The design and development of new materials rely heavily on understanding the relationship between structure and properties. Engineers select materials with specific properties for various applications based on their mechanical strength, durability, conductivity, and other characteristics.
-
Chemistry: The study of crystal structures and bonding in solids provides insights into the chemical behavior of matter.
-
Physics: Solid-state physics explores the fundamental properties of solids, including their electronic, magnetic, and optical behavior.
-
Geology: The properties of minerals and rocks are crucial in understanding geological processes and the Earth's structure.
-
Medicine: The properties of biomaterials, such as implants and prosthetics, are critical in biomedical applications.
Conclusion
The world of solids is incredibly diverse, with an extensive range of properties stemming from the intricate interplay of their atomic structure, bonding, and crystallographic arrangement. From the hardness of diamond to the malleability of gold, the characteristics of solids dictate their use in countless applications, highlighting the importance of understanding their fundamental properties across numerous scientific and engineering disciplines. Continued research into the properties of solids promises further advancements in materials science, leading to innovative solutions across diverse technological sectors.
Latest Posts
Latest Posts
-
Example Of An Adjusted Trial Balance
Apr 07, 2025
-
Who Is Credited With First Observing Cells
Apr 07, 2025
-
How To Write An Equation Of A Vertical Line
Apr 07, 2025
-
What Is The Building Block Monomer For A Nucleic Acids
Apr 07, 2025
-
Where Is The Serous Membrane Located
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Are The Properties Of Solids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
