What Are The Three Main Varibles Of The Gas Laws
Muz Play
Mar 16, 2025 · 6 min read
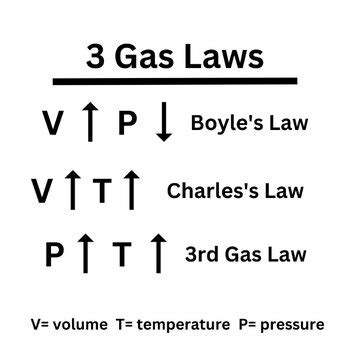
Table of Contents
What Are the Three Main Variables of the Gas Laws?
Understanding the behavior of gases is crucial in numerous scientific fields, from atmospheric science and chemistry to engineering and medicine. This understanding is largely built upon the gas laws, which describe the relationships between several key variables that define the state of a gas. While several gas laws exist, each focusing on a specific relationship, the three main variables that underpin all of them are pressure (P), volume (V), and temperature (T). This article will delve deep into each of these variables, explaining their significance in the gas laws and how they interact with each other.
Pressure (P): The Force Exerted by Gas Molecules
Pressure is defined as the force exerted per unit area. In the context of gases, this force originates from the constant, random motion of gas molecules. As these molecules collide with the walls of their container, they exert a force. The more frequent and forceful these collisions, the higher the pressure.
Units of Pressure
Pressure is measured in a variety of units, with the most common including:
- Pascals (Pa): The SI unit of pressure, defined as one newton per square meter (N/m²).
- Atmospheres (atm): A unit based on standard atmospheric pressure at sea level, approximately 101,325 Pa.
- Torr (mmHg): A unit based on the height of a mercury column in a barometer, equivalent to 1/760 atm.
- Pounds per square inch (psi): A common unit in engineering applications.
The choice of unit often depends on the context of the application or experiment. Understanding the conversion factors between these units is crucial for accurate calculations involving gas laws.
Factors Affecting Pressure
Several factors influence the pressure exerted by a gas:
- Number of gas molecules: A larger number of gas molecules leads to more frequent collisions and therefore higher pressure. This is directly proportional; doubling the number of molecules roughly doubles the pressure, assuming other variables remain constant.
- Temperature: Higher temperatures result in faster-moving molecules, leading to more forceful collisions and increased pressure. This relationship is directly proportional as well.
- Volume: Decreasing the volume of a container forces the gas molecules closer together, increasing the frequency of collisions and raising the pressure. Conversely, increasing the volume reduces the pressure. This relationship is inversely proportional.
Understanding how these factors influence pressure is essential for interpreting the results of gas law experiments and predicting the behavior of gases under various conditions.
Volume (V): The Space Occupied by a Gas
Volume refers to the amount of three-dimensional space occupied by a gas. It's directly influenced by the number of gas molecules and their movement. Unlike solids and liquids, gases expand to fill the available space of their container.
Units of Volume
Volume is typically measured in:
- Liters (L): A common unit in chemistry and related fields.
- Cubic meters (m³): The SI unit of volume.
- Cubic centimeters (cm³): Often used for smaller volumes.
- Milliliters (mL): Another common unit, especially in laboratory settings.
Again, the choice of unit depends on the specific application and the scale of the volume being measured.
Factors Affecting Volume
The volume of a gas is primarily determined by:
- Number of gas molecules: More gas molecules occupy a larger volume, assuming constant pressure and temperature. This is a directly proportional relationship.
- Temperature: Increasing the temperature increases the kinetic energy of the gas molecules, causing them to move faster and occupy a larger volume (at constant pressure). This is a directly proportional relationship.
- Pressure: Reducing the pressure on a gas allows it to expand, increasing its volume. Conversely, increasing the pressure compresses the gas, reducing its volume. This is an inversely proportional relationship.
Temperature (T): The Average Kinetic Energy of Gas Molecules
Temperature is a measure of the average kinetic energy of the gas molecules. Kinetic energy is the energy of motion, and higher temperatures indicate that the gas molecules are moving faster and possess more kinetic energy.
Units of Temperature
Temperature is measured in:
- Kelvin (K): The SI unit of temperature, an absolute scale where 0 K represents absolute zero – the theoretical point where all molecular motion ceases.
- Celsius (°C): A widely used scale, with 0 °C representing the freezing point of water and 100 °C representing the boiling point of water at standard pressure.
- Fahrenheit (°F): A less common scale in scientific contexts.
It's crucial to use the Kelvin scale for calculations involving gas laws because it accounts for the absolute value of kinetic energy. Conversions between these scales are necessary to ensure accurate calculations.
Factors Affecting Temperature
Temperature directly affects the kinetic energy of gas molecules and, consequently, their behavior:
- Pressure: At constant volume, increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and forceful collisions with the container walls and a higher pressure.
- Volume: At constant pressure, increasing the temperature increases the volume occupied by the gas as molecules move faster and spread out.
- Number of moles: The number of moles doesn't directly affect the temperature itself, but the total kinetic energy of the system will be higher with more moles at a given temperature.
The interplay between temperature, pressure, and volume forms the foundation of various gas laws.
The Interplay of Pressure, Volume, and Temperature: The Ideal Gas Law
The relationship between pressure, volume, and temperature is elegantly summarized in the Ideal Gas Law:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas (amount of substance)
- R is the ideal gas constant (a proportionality constant that depends on the units used)
- T is the absolute temperature of the gas (in Kelvin)
The Ideal Gas Law is a powerful tool that allows us to predict the behavior of gases under various conditions, assuming they behave ideally (which means the gas molecules don't significantly interact with each other).
This law incorporates all three key variables: pressure, volume, and temperature, and shows how they are interlinked. Changes in one variable will inevitably affect the others, keeping the overall equation balanced.
Real Gases vs. Ideal Gases
It’s important to note that the Ideal Gas Law is a simplification. Real gases deviate from ideal behavior, especially at high pressures and low temperatures. This is because real gas molecules do possess intermolecular forces and occupy a small but non-negligible volume themselves. These interactions can significantly affect the pressure and volume of the gas. More sophisticated equations of state, such as the Van der Waals equation, are necessary to accurately model the behavior of real gases under non-ideal conditions.
Conclusion
Pressure, volume, and temperature are the three cornerstone variables that dictate the behavior of gases. Understanding their individual definitions, units, and how they influence each other is fundamental to comprehending the gas laws. The Ideal Gas Law serves as a powerful model for predicting gas behavior, providing a strong foundation for further exploration of thermodynamics and related fields. While the Ideal Gas Law provides a useful approximation, it is crucial to remember its limitations and to be aware of when deviations from ideal behavior become significant. By fully grasping the interactions of these three variables, we can accurately predict and manipulate the properties of gases in a wide range of scientific and engineering applications.
Latest Posts
Latest Posts
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Main Varibles Of The Gas Laws . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
