What Are The Vertical Columns Called In The Periodic Table
Muz Play
Mar 27, 2025 · 6 min read
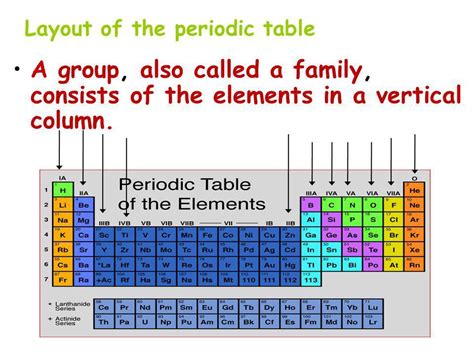
Table of Contents
What Are the Vertical Columns Called in the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes chemical elements based on their atomic number, electron configurations, and recurring chemical properties. Understanding its structure is crucial for comprehending the behavior of elements and their interactions. While the horizontal rows are known as periods, the vertical columns are called groups (or sometimes families). This article will delve deep into the significance of these groups, exploring their properties, trends, and the fascinating history behind their organization.
Understanding Groups: A Deeper Dive into Vertical Columns
The elements within a group share similar chemical properties due to having the same number of valence electrons. Valence electrons are the electrons in the outermost shell of an atom, which are primarily responsible for chemical bonding and reactivity. Since elements in the same group possess the same number of valence electrons, they tend to exhibit similar bonding patterns and participate in similar chemical reactions.
For example, the alkali metals (Group 1) all have one valence electron, making them highly reactive and readily forming +1 ions. Similarly, the halogens (Group 17) have seven valence electrons, leading to their high reactivity and tendency to form -1 ions. This consistent behavior across a group simplifies predicting the chemical behavior of elements based on their group placement.
The Significance of Electron Configuration
The similarity in chemical properties within a group is directly linked to their electron configuration. The electron configuration describes how electrons are arranged in energy levels and subshells within an atom. Elements in the same group have similar electron configurations in their outermost shell, resulting in similar chemical behavior.
This principle holds true regardless of the element's period (row). While elements in the same period have different numbers of valence electrons and, consequently, different chemical properties, elements in the same group display remarkable similarities. Understanding this relationship between electron configuration, valence electrons, and group properties is critical for mastering the periodic table.
Exploring the Different Groups: A Detailed Overview
The periodic table's 18 groups are not all equal in their significance or complexity. Some groups contain elements with vastly different properties, while others exhibit striking similarities. Let's explore some key groups:
Group 1: Alkali Metals
The alkali metals are highly reactive metals characterized by their single valence electron. This electron is easily lost, forming +1 ions and resulting in their high reactivity. They are soft, silvery-white metals with low melting points. Examples include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Their reactivity increases as you go down the group.
Group 2: Alkaline Earth Metals
These metals also exhibit high reactivity, though slightly less than the alkali metals. They have two valence electrons, readily forming +2 ions. They are harder, denser, and have higher melting points than the alkali metals. Examples include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Group 17: Halogens
The halogens are highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions, creating stable halide ions. They exist as diatomic molecules (e.g., Cl₂, Br₂) under standard conditions. Examples include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Their reactivity decreases as you go down the group.
Group 18: Noble Gases
The noble gases are unique in their inertness. They have a full valence shell (eight electrons, except for helium with two), making them very stable and unreactive. They are colorless, odorless gases under standard conditions. Examples include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Transition Metals (Groups 3-12)
The transition metals occupy the central block of the periodic table. They are characterized by their variable oxidation states and the formation of colored compounds. Their properties are less predictable than those of the main group elements due to the involvement of d-electrons in bonding. This group includes many familiar metals like iron (Fe), copper (Cu), gold (Au), and platinum (Pt).
Inner Transition Metals (Lanthanides and Actinides)
Located below the main body of the periodic table, the lanthanides and actinides are characterized by the filling of the 4f and 5f orbitals, respectively. These elements exhibit similar chemical properties within their series due to the shielding effect of the electrons in the inner orbitals. Many of the actinides are radioactive.
Trends Across Groups: Periodic Properties
Various periodic properties exhibit trends as you move down a group. Understanding these trends helps predict the behavior of elements. Some key trends include:
- Atomic Radius: Generally increases down a group due to the addition of electron shells.
- Ionization Energy: Generally decreases down a group as the outermost electrons are further from the nucleus and experience less attraction.
- Electronegativity: Generally decreases down a group as the outermost electrons are further from the nucleus and less strongly attracted.
- Metallic Character: Generally increases down a group, with elements becoming more metallic in their properties.
Historical Context: The Evolution of Group Organization
The organization of the periodic table into groups wasn't a single event but a gradual process, reflecting our growing understanding of atomic structure and chemical behavior. Early attempts at classification focused on atomic weights and recurring properties. Dmitri Mendeleev's 1869 periodic table is considered a landmark achievement, arranging elements by atomic weight and predicting the properties of undiscovered elements. However, the modern periodic table, based on atomic number and electron configuration, provides a more accurate and comprehensive representation of chemical elements and their relationships.
Beyond the Basic Groups: Subgroups and Anomalies
While the 18-group system is widely used, some nuances deserve consideration:
- Subgroups: Within some groups, further subdivisions (subgroups) can be identified based on subtle differences in chemical behavior. This is particularly relevant for transition metals.
- Anomalous Behavior: Some elements deviate slightly from expected group trends due to factors such as electron-electron interactions or relativistic effects. These anomalies highlight the complexity of atomic structure and the limitations of simple generalizations.
The Importance of Understanding Groups in Chemistry
The concept of groups is fundamental to understanding chemistry. It provides a framework for:
- Predicting chemical properties: Knowing the group of an element allows chemists to predict its reactivity, bonding behavior, and other chemical characteristics.
- Designing chemical reactions: This knowledge is crucial for designing and controlling chemical reactions, which is vital in many applications, from industrial processes to drug discovery.
- Developing new materials: Understanding group trends enables the development of new materials with specific properties, tailored for particular applications.
Conclusion
The vertical columns of the periodic table, known as groups or families, are not mere organizational features but are crucial for understanding the fundamental principles of chemistry. The similarities in valence electron configuration within a group lead to predictable trends in chemical properties, allowing us to infer and predict the behavior of elements. Understanding the properties of various groups – from the highly reactive alkali metals to the inert noble gases – is essential for anyone seeking to master chemistry. The evolution of the periodic table and the ongoing refinement of our understanding of group behavior highlight the power of scientific inquiry and the ever-evolving nature of our knowledge in this field. The periodic table, with its groups and periods, continues to serve as a powerful tool and a testament to the beauty and elegance of the chemical world.
Latest Posts
Latest Posts
-
A Tentative Explanation For An Event Which Can Be Testabl
Mar 30, 2025
-
How To Check If A Matrix Is Invertible
Mar 30, 2025
-
Crystal Field Theory Energy Level Diagram
Mar 30, 2025
-
Blood Agar Plate Selective Or Differential
Mar 30, 2025
-
Organic Compounds Contain Atoms Of What Element
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Are The Vertical Columns Called In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
