What Does A Negative Delta H Mean
Muz Play
Mar 25, 2025 · 6 min read
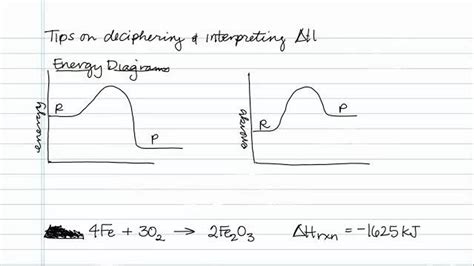
Table of Contents
What Does a Negative Delta H Mean? Understanding Enthalpy Change
Understanding thermodynamics is crucial in various scientific fields, from chemistry and physics to engineering and environmental science. A key concept within thermodynamics is enthalpy, and its change, represented by ΔH, plays a vital role in describing the energy changes during a chemical or physical process. This article delves into the meaning of a negative ΔH, exploring its implications, providing real-world examples, and clarifying common misconceptions.
Enthalpy: A Measure of System Energy
Before diving into the significance of a negative ΔH, let's establish a clear understanding of enthalpy itself. Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's not a directly measurable quantity but rather a state function, meaning its value depends only on the system's current state, not the path taken to reach that state. Think of it as the total energy stored within a system, including its internal energy and the energy associated with pressure-volume work.
Key takeaway: Enthalpy is a measure of the total heat content of a system under constant pressure.
Delta H (ΔH): The Change in Enthalpy
The change in enthalpy, denoted as ΔH, represents the difference in enthalpy between the final and initial states of a system undergoing a process. It's calculated as:
ΔH = H<sub>final</sub> - H<sub>initial</sub>
ΔH provides crucial information about the heat transfer during a process. A positive ΔH indicates an endothermic process, where the system absorbs heat from its surroundings. Conversely, a negative ΔH signifies an exothermic process, where the system releases heat into its surroundings.
Deciphering a Negative Delta H: Exothermic Reactions
A negative ΔH is the hallmark of an exothermic reaction or process. In these processes, the energy released as heat is greater than the energy absorbed. This excess energy is transferred to the surroundings, causing a temperature increase in the system's environment. The magnitude of the negative ΔH indicates the amount of heat released. A larger negative value implies a greater release of heat.
Key takeaway: A negative ΔH means the reaction releases heat to its surroundings; it's an exothermic process.
Examples of Exothermic Processes with Negative ΔH
Many everyday phenomena involve exothermic reactions with negative ΔH. Here are some prominent examples:
1. Combustion Reactions:
Combustion, the rapid oxidation of a substance, is a highly exothermic process. Burning fuels like wood, natural gas (methane), propane, and gasoline releases significant amounts of heat. The negative ΔH values for these reactions are substantial, making them valuable sources of energy. For example, the combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) ΔH < 0
The negative ΔH signifies the large amount of heat released during the burning of methane.
2. Neutralization Reactions:
The reaction between an acid and a base to form salt and water is another common exothermic process. The heat released during neutralization is often used to determine the enthalpy of neutralization. For instance, the neutralization of a strong acid like hydrochloric acid (HCl) with a strong base like sodium hydroxide (NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) ΔH < 0
The negative ΔH in this reaction highlights the heat released upon mixing the acid and base.
3. Formation of Chemical Bonds:
The formation of chemical bonds generally involves the release of energy. This is because electrons are drawn closer together, leading to a more stable arrangement and the release of excess energy in the form of heat. The stronger the bonds formed, the more negative the ΔH will be.
4. Condensation:
Phase transitions can also have associated enthalpy changes. When a substance changes from a gas to a liquid (condensation), the molecules lose kinetic energy and come closer together, releasing heat. Therefore, the enthalpy change of condensation is always negative.
5. Freezing:
Similar to condensation, freezing (liquid to solid) is an exothermic process. As a liquid freezes, its molecules become more ordered, releasing energy in the form of heat. This is why ice releases heat as it forms.
Applications of Exothermic Reactions (Negative ΔH)
The understanding and application of exothermic reactions (negative ΔH) are widespread across numerous fields:
- Energy Production: Combustion of fuels remains the primary source of energy for electricity generation and transportation.
- Industrial Processes: Many industrial processes leverage exothermic reactions, utilizing the generated heat either directly or indirectly.
- Heating and Cooling Systems: Exothermic reactions can be used in the development of efficient heating systems.
- Chemical Synthesis: Many chemical synthesis reactions are exothermic, providing a driving force for product formation.
- Materials Science: Understanding heat released during material formation (like curing of resins or cement hardening) is crucial for controlling material properties.
Misconceptions about Negative Delta H
Several misconceptions surround negative ΔH. It's crucial to clarify these to ensure a proper understanding:
- Negative ΔH does not imply a fast reaction: The speed of a reaction (kinetics) is independent of its enthalpy change (thermodynamics). A reaction can have a very negative ΔH but still proceed slowly.
- Negative ΔH does not guarantee spontaneity: While a negative ΔH favors a reaction, spontaneity depends on both enthalpy and entropy changes (Gibbs Free Energy). A reaction with a negative ΔH but a positive and large enough ΔS can still be nonspontaneous.
- Negative ΔH doesn't indicate the extent of reaction: The negative value of ΔH tells only about the heat released, not the amount of reactants that have converted into products at equilibrium.
Beyond Just Heat: The Importance of Entropy
While enthalpy change (ΔH) provides valuable insights into the heat transfer, the overall spontaneity of a process is governed by the Gibbs Free Energy (ΔG), which considers both enthalpy and entropy changes. The equation for Gibbs Free Energy is:
ΔG = ΔH - TΔS
Where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy
- T is the absolute temperature
- ΔS is the change in entropy
Entropy (S) measures the randomness or disorder of a system. A positive ΔS indicates an increase in disorder, while a negative ΔS signifies a decrease. A negative ΔH favors spontaneity, but a positive ΔS also contributes to spontaneity. At low temperatures, a negative ΔH is more significant; while at high temperatures, a positive ΔS plays a more dominant role.
Conclusion: A Negative ΔH – A Powerful Indicator
A negative ΔH definitively indicates an exothermic process – one that releases heat into its surroundings. This fundamental concept is crucial in various fields, from understanding energy production to designing efficient chemical processes. While a negative ΔH contributes to the spontaneity of a process, remember that the overall spontaneity is dictated by the Gibbs Free Energy, which encompasses both enthalpy and entropy changes. Understanding this interplay is essential for a comprehensive grasp of thermodynamic principles and their applications.
Latest Posts
Latest Posts
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 27, 2025
-
Claim Of Fact Value And Policy
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Does A Negative Delta H Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
