What Does Atomic Number Of An Element Represent
Muz Play
Mar 31, 2025 · 5 min read
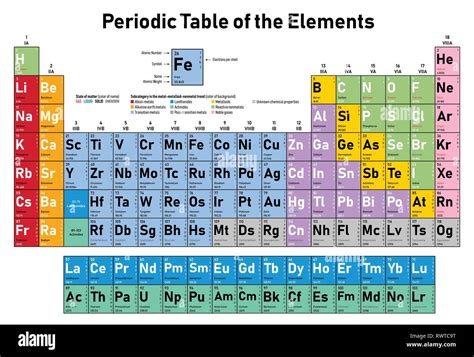
Table of Contents
What Does the Atomic Number of an Element Represent?
The atomic number of an element, a fundamental concept in chemistry and physics, holds the key to understanding its identity, properties, and behavior. It's more than just a number; it's a defining characteristic that dictates how an element interacts with other elements and forms compounds. This article delves deep into the meaning and significance of the atomic number, exploring its connection to protons, electrons, and the periodic table, and demonstrating its importance in various scientific disciplines.
The Atomic Number: A Defining Characteristic
The atomic number of an element is simply the number of protons found in the nucleus of each atom of that element. This is a crucial distinction: it's not the number of protons in a nucleus, but the number of protons in every atom of a specific element. This consistency is what defines an element. For example, every atom of hydrogen has one proton, giving it an atomic number of 1. Every atom of oxygen has eight protons, giving it an atomic number of 8. This number remains constant regardless of the number of neutrons or electrons present.
Isotopes and the Invariance of Atomic Number
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For instance, carbon-12 (¹²C) and carbon-14 (¹⁴C) are both isotopes of carbon. They both have six protons (atomic number 6), but ¹²C has six neutrons while ¹⁴C has eight. Despite this difference in neutron count, both are still carbon because their atomic number remains the same. This highlights the pivotal role of the atomic number in elemental identity. The atomic number is unchanging; it's the bedrock upon which an element is built.
The Atomic Number and the Periodic Table
The periodic table, a cornerstone of chemistry, is organized primarily based on the atomic numbers of elements. Elements are arranged in ascending order of their atomic numbers, revealing periodic trends in their properties. This arrangement is not arbitrary; it reflects the underlying electronic structure of atoms, which is directly determined by the number of protons. The periodic table's structure allows scientists to predict the properties of elements based on their position and relationships with neighboring elements.
Groups and Periods: Reflections of Electronic Structure
The groups (vertical columns) on the periodic table reflect elements with similar chemical properties. This similarity arises from the same number of electrons in their outermost shell, a number directly influenced by the element's atomic number. The periods (horizontal rows) represent elements with the same number of electron shells, again a consequence of the sequential increase in atomic number. This systematic arrangement allows for a powerful predictive tool in understanding chemical reactivity and behavior.
Beyond the Nucleus: Electrons and Atomic Number
While the atomic number is defined by the protons in the nucleus, it has a profound influence on the number of electrons in a neutral atom. In a neutral atom, the number of electrons equals the number of protons. This balance of positive and negative charges results in a net neutral electrical charge. This electron configuration dictates the chemical behavior of the element, determining its reactivity, bonding capacity, and the types of compounds it can form.
Valence Electrons and Chemical Reactivity
The valence electrons, located in the outermost electron shell, are particularly important in determining chemical reactivity. The number of valence electrons is directly related to the atomic number and influences an element's tendency to gain, lose, or share electrons to achieve a stable electron configuration (often a full outermost shell). Elements in the same group have similar valence electron configurations, explaining their similar chemical behaviors. Understanding valence electrons, inherently linked to the atomic number, is essential for comprehending chemical bonding and reactions.
Applications of Atomic Number in Various Fields
The significance of the atomic number extends far beyond the realm of basic chemistry. Its applications span numerous scientific fields, including:
1. Nuclear Physics and Radioactivity:
The atomic number plays a crucial role in understanding nuclear reactions and radioactive decay. It helps identify the parent and daughter nuclides in radioactive decay processes. Knowing the atomic number allows physicists to predict the type of radiation emitted and the resulting change in the atomic number of the daughter nuclide. This is critical in nuclear medicine, nuclear power generation, and radioisotope dating.
2. Spectroscopy and Elemental Analysis:
Atomic number is essential in various spectroscopic techniques, which analyze the interaction of electromagnetic radiation with matter. Each element has a unique spectral signature, determined by its electron configuration, which is in turn directly linked to its atomic number. Spectroscopic methods are used to identify and quantify elements in various samples, including environmental monitoring, materials science, and forensic science.
3. Astrophysics and Cosmology:
The atomic numbers of elements are fundamental in understanding the origin and evolution of the universe. The abundances of elements in stars and other celestial bodies provide crucial clues about stellar nucleosynthesis, the process by which elements are created in stars. By analyzing the light emitted by stars, astronomers can determine the elemental composition, identifying elements based on their characteristic spectral lines, which are intimately connected to their atomic numbers.
4. Materials Science and Engineering:
The atomic number influences the physical and chemical properties of materials. Understanding the atomic number of constituent elements is crucial in designing materials with specific properties. For example, the atomic number helps predict the strength, conductivity, and other properties of alloys and semiconductors. This knowledge is fundamental in developing advanced materials for various applications, including aerospace engineering, electronics, and biomedicine.
Conclusion: The Ubiquity of Atomic Number
The atomic number is far more than a simple identifier; it's the fundamental characteristic that underpins the properties and behavior of each element. From defining the identity of an element to influencing its chemical reactivity and dictating its position on the periodic table, the atomic number plays a crucial role in chemistry, physics, and numerous related fields. Its significance extends to understanding the universe's composition, developing new materials, and analyzing various substances. Understanding the atomic number is therefore essential for any serious study of the natural world. The power and importance of this single number cannot be overstated. Its consistent presence throughout scientific inquiry solidifies its position as a cornerstone of modern science.
Latest Posts
Latest Posts
-
Unity Of Life And Diversity Of Life
Apr 01, 2025
-
Choose The Most Likely Correlation Value For This Scatterplot
Apr 01, 2025
-
Which Is The Noble Gas Notation For Chlorine
Apr 01, 2025
-
Describe The Role Of Carbon In Biological Systems
Apr 01, 2025
-
How Is The Domain Rank Different From Other Ranks
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Does Atomic Number Of An Element Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
