What Does It Mean When A Reaction Is Spontaneous
Muz Play
Mar 26, 2025 · 6 min read
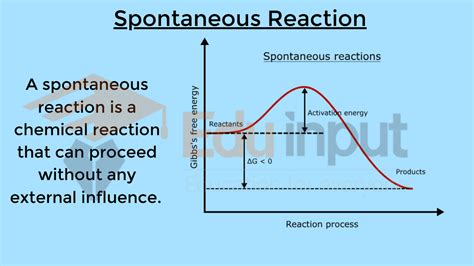
Table of Contents
What Does it Mean When a Reaction is Spontaneous? Understanding Gibbs Free Energy and Reaction Spontaneity
Spontaneity in chemistry refers to whether a reaction will proceed without external intervention. It doesn't indicate how fast the reaction occurs, only whether it will happen at all under a given set of conditions. A spontaneous reaction can be incredibly slow, while a non-spontaneous reaction might be forced to occur with the input of energy. Understanding spontaneity is crucial in various fields, from predicting chemical reactions to designing efficient energy systems. This article delves deep into the concept of spontaneous reactions, explaining the underlying principles and factors that determine their occurrence.
Gibbs Free Energy: The Deciding Factor
The key to understanding spontaneity lies in Gibbs Free Energy (G), a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. The change in Gibbs Free Energy (ΔG) during a reaction is the ultimate indicator of spontaneity.
-
ΔG < 0 (Negative): The reaction is spontaneous under the given conditions. The system will proceed in the forward direction without external intervention. This means the products are more stable than the reactants.
-
ΔG > 0 (Positive): The reaction is non-spontaneous under the given conditions. The reaction will not proceed in the forward direction without external input of energy (e.g., heat, electricity). The reactants are more stable than the products.
-
ΔG = 0 (Zero): The reaction is at equilibrium. The rates of the forward and reverse reactions are equal, and there is no net change in the concentrations of reactants or products.
The Relationship Between Enthalpy, Entropy, and Gibbs Free Energy
Gibbs Free Energy is linked to two other crucial thermodynamic quantities: enthalpy (H) and entropy (S). The relationship is expressed by the following equation:
ΔG = ΔH - TΔS
Where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content)
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy (disorder)
Let's break down the individual components:
-
Enthalpy (ΔH): Represents the heat absorbed or released during a reaction at constant pressure. An exothermic reaction (ΔH < 0) releases heat, while an endothermic reaction (ΔH > 0) absorbs heat. Exothermic reactions tend to be favored because they release energy, increasing stability.
-
Entropy (ΔS): Measures the degree of disorder or randomness in a system. An increase in entropy (ΔS > 0) indicates a more disordered state, while a decrease in entropy (ΔS < 0) indicates a more ordered state. Systems naturally tend towards greater disorder (higher entropy).
The equation ΔG = ΔH - TΔS reveals that spontaneity is a balance between enthalpy and entropy. Let's analyze the different scenarios:
Scenarios Based on Enthalpy and Entropy Changes
-
ΔH < 0 and ΔS > 0: This is the most favorable scenario. The reaction is exothermic (releases heat) and increases disorder. ΔG will always be negative, making the reaction spontaneous at all temperatures.
-
ΔH > 0 and ΔS < 0: This is the least favorable scenario. The reaction is endothermic (absorbs heat) and decreases disorder. ΔG will always be positive, making the reaction non-spontaneous at all temperatures.
-
ΔH < 0 and ΔS < 0: The reaction is exothermic, but it decreases disorder. In this case, spontaneity depends on the temperature. At low temperatures, the negative ΔH term dominates, making ΔG negative and the reaction spontaneous. At high temperatures, the positive TΔS term can outweigh the negative ΔH, making ΔG positive and the reaction non-spontaneous.
-
ΔH > 0 and ΔS > 0: The reaction is endothermic and increases disorder. Similar to the previous case, spontaneity depends on temperature. At low temperatures, the positive ΔH term dominates, making ΔG positive and the reaction non-spontaneous. At high temperatures, the positive TΔS term can outweigh the positive ΔH, making ΔG negative and the reaction spontaneous.
Factors Affecting Spontaneity
Beyond enthalpy and entropy, several other factors can influence the spontaneity of a reaction:
-
Temperature: As seen in the Gibbs Free Energy equation, temperature plays a crucial role, especially when ΔH and ΔS have opposing signs. Raising the temperature can shift the balance and make a previously non-spontaneous reaction spontaneous (or vice-versa).
-
Pressure: Changes in pressure primarily affect reactions involving gases. Increasing pressure generally favors reactions that produce fewer gas molecules, while decreasing pressure favors reactions that produce more gas molecules.
-
Concentration: The concentrations of reactants and products affect the reaction quotient (Q), which is related to ΔG through the equation: ΔG = ΔG° + RTlnQ, where ΔG° is the standard Gibbs Free Energy change, R is the gas constant, and T is the temperature. Higher reactant concentrations generally drive the reaction forward.
-
Catalysts: Catalysts speed up the rate of a reaction but do not affect the equilibrium position or the spontaneity of the reaction. They lower the activation energy, making it easier for the reaction to proceed, but they do not change the overall ΔG.
Examples of Spontaneous and Non-Spontaneous Reactions
Spontaneous Reactions:
-
Rusting of iron: The oxidation of iron in the presence of oxygen and water is a spontaneous process. It's exothermic (ΔH < 0) and leads to an increase in entropy (ΔS > 0) due to the formation of a more disordered iron oxide.
-
Combustion of fuels: Burning fuels like wood or gasoline is a spontaneous reaction. It's highly exothermic and leads to a significant increase in entropy as the reactants are converted into numerous gaseous products.
-
Dissolution of salts in water: Many salts readily dissolve in water, a spontaneous process driven by favorable enthalpy and entropy changes. The interaction between water molecules and ions is energetically favorable (negative ΔH), and the increased disorder of the ions in solution contributes to a positive ΔS.
Non-Spontaneous Reactions:
-
Decomposition of water into hydrogen and oxygen: This reaction requires significant energy input (electrolysis) to occur. It's endothermic (ΔH > 0) and leads to a decrease in entropy (ΔS < 0) as the highly ordered gas molecules are separated from the liquid phase.
-
Formation of diamond from graphite: Graphite is the more stable form of carbon under standard conditions. The conversion to diamond is non-spontaneous under normal circumstances. It requires high pressure and temperature to force the reaction forward.
-
Melting ice at temperatures below 0°C: Ice melts spontaneously above 0°C because the process is accompanied by an increase in entropy that outweighs the energy needed to break the ice's crystal structure. However, below 0°C, the process is non-spontaneous because the enthalpy increase is too large.
Spontaneity and Equilibrium
It's crucial to understand that spontaneity only indicates whether a reaction will proceed towards equilibrium. It doesn't imply that the reaction will go to completion. Even spontaneous reactions reach equilibrium, at which point the forward and reverse reaction rates are equal, and there is no further net change in the concentrations of reactants and products. The position of equilibrium is determined by the magnitude of ΔG°. A larger negative ΔG° indicates that the equilibrium lies far to the right (favoring products).
Conclusion
Understanding spontaneity is fundamental to comprehending chemical reactions and their behavior. While the Gibbs Free Energy change (ΔG) is the ultimate determinant, the interplay of enthalpy (ΔH) and entropy (ΔS) dictates whether a reaction will proceed spontaneously under given conditions. Considering factors like temperature, pressure, and concentration further refines our prediction of reaction spontaneity. By mastering this concept, you gain a powerful tool for analyzing chemical systems and predicting their behavior. This knowledge is indispensable in various fields, including materials science, chemical engineering, and environmental science, among others.
Latest Posts
Latest Posts
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
-
What Are The Vertical Columns Called On A Periodic Table
Mar 29, 2025
-
Where Does Fermentation Take Place In A Cell
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Does It Mean When A Reaction Is Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
